

CN 11-4766/R
主办:中国科学院心理研究所
出版:科学出版社


心理科学进展 ›› 2024, Vol. 32 ›› Issue (7): 1031-1047.doi: 10.3724/SP.J.1042.2024.01031 cstr: 32111.14.2024.01031
• 研究构想 • 下一篇
收稿日期:2023-10-27
出版日期:2024-07-15
发布日期:2024-05-09
通讯作者:
刘威, E-mail: weiliu1991@ccnu.edu.cn基金资助:
LIU Wei( ), CHEN Ruixin, GUO JinPeng
), CHEN Ruixin, GUO JinPeng
Received:2023-10-27
Online:2024-07-15
Published:2024-05-09
摘要:
记忆巩固通常在记忆编码后的休息或睡眠期间缓慢发生。然而, 在应激状态下, 记忆有可能被快速巩固。鉴于长期以来缺乏对人类记忆巩固期神经活动的量化方法, 应激状态下记忆快速巩固的机制尚未明确。本研究拟采用计算神经科学手段, 详细刻画应激状态下人类情景记忆巩固期的神经重放过程。此外, 我们还将整合认知心理学、脑成像技术、机器学习、神经内分泌调控、应激诱发及生理生化检测等跨学科方法, 来验证应激对神经重放的“双刃剑”效应假说: 尽管应激可能会加快神经重放的速度, 促进记忆巩固, 但它同时也可能会降低神经重放的准确性并干扰其顺序。本研究将: (1)比较应激和非应激状态下神经重放的多维特征差异; (2)探寻应激状态下神经重放与记忆提取和编码的交互作用; (3)尝试利用神经内分泌和环境策略来调控人类的应激反应, 进而影响神经重放。本研究能够有助于确定促进记忆巩固的理想大脑状态, 并整合人类和动物的神经重放研究。同时, 本研究还可能为保护应激状态下的情景记忆功能, 以及干预应激类精神疾病中的记忆障碍提供全新策略。
中图分类号:
刘威, 陈瑞欣, 郭金朋. (2024). 应激下人类情景记忆巩固的神经重放机制. 心理科学进展 , 32(7), 1031-1047.
LIU Wei, CHEN Ruixin, GUO JinPeng. (2024). The neural replay mechanisms of episodic memory consolidation under stress in humans. Advances in Psychological Science, 32(7), 1031-1047.
| 研究 | 样本量 | 被试 | 任务 | 应激诱发 | 间隔1 | 结果 | |
|---|---|---|---|---|---|---|---|
| 应激组 | 控制组 | ||||||
| Zinkin & Miller, | 40 | 34 | 大鼠 | a single avoidance learning | electroconvulsive shock | 24 h, 48 h, 72 h | 增强 |
| Diamond & Rose, | / | 大鼠 | maze training paradigm | recording chamber | 4 h | 损害 | |
| Dornelles et al., | / | 大鼠 | a novel object recognition | epinephrine | 96 h | 增强 | |
| Roozendaal et al., | 20 | 13 | 大鼠 | object recognition training | intra-BLA infusions of norepinephrine | 24 h | 倒U |
| Campolongo et al., | 10-11 | 10-11 | 大鼠 | inhibitory avoidance | The CB1 receptor agonist | 48 h | 增强 |
| Bass et al., | 9 | 大鼠 | a novel object recognition | direct activation of the BLA | 1 d | 增强 | |
| Barsegyan et al., | 39 | 13 | 大鼠 | object-in-context recognition | intra-BLA infusions of norepinephrine | 24 h | 倒U |
| McReynolds et al., | 7 | 6 | 大鼠 | inhibitory avoidance, object recognition | intra-BLA infusions of clenbuterol | 48 h | 增强 |
| Morena et al., | 10-14 | 10-14 | 大鼠 | inhibitory avoidance | fatty acid amide hydrolase inhibitor | 48 h | 增强 |
| Siller-Pérez et al., | 20 | 11 | 大鼠 | inhibitory avoidance | WIN55, 212-2 into the dorsal striatum | 48 h | 增强 |
| Cahill et al., | 25 | 23 | 人类 | slides of varying emotional content | cold pressor stress | 1 wk | 增强 |
| van Marle et al., | 20 | 19 | 人类 | negative and neutral pictures | administered hydrocortisone during sleep | 1 d | 增强 |
| Borota et al., | 35 | 38 | 人类 | studied images of objects | 200 mg of caffeine | 24 h | 增强 |
| McCullough et al., | 23 | 24 | 人类 | emotional and neutral pictures | cold-pressor test | 24 h | 倒U |
| Krenz et al., | 52 | 52 | 人类 | negative and neutral pictures | α2-adrenoceptor antagonist yohimbine | 1 d, 28 d | 增强 |
表1 应激下的记忆巩固: 增强还是减弱?
| 研究 | 样本量 | 被试 | 任务 | 应激诱发 | 间隔1 | 结果 | |
|---|---|---|---|---|---|---|---|
| 应激组 | 控制组 | ||||||
| Zinkin & Miller, | 40 | 34 | 大鼠 | a single avoidance learning | electroconvulsive shock | 24 h, 48 h, 72 h | 增强 |
| Diamond & Rose, | / | 大鼠 | maze training paradigm | recording chamber | 4 h | 损害 | |
| Dornelles et al., | / | 大鼠 | a novel object recognition | epinephrine | 96 h | 增强 | |
| Roozendaal et al., | 20 | 13 | 大鼠 | object recognition training | intra-BLA infusions of norepinephrine | 24 h | 倒U |
| Campolongo et al., | 10-11 | 10-11 | 大鼠 | inhibitory avoidance | The CB1 receptor agonist | 48 h | 增强 |
| Bass et al., | 9 | 大鼠 | a novel object recognition | direct activation of the BLA | 1 d | 增强 | |
| Barsegyan et al., | 39 | 13 | 大鼠 | object-in-context recognition | intra-BLA infusions of norepinephrine | 24 h | 倒U |
| McReynolds et al., | 7 | 6 | 大鼠 | inhibitory avoidance, object recognition | intra-BLA infusions of clenbuterol | 48 h | 增强 |
| Morena et al., | 10-14 | 10-14 | 大鼠 | inhibitory avoidance | fatty acid amide hydrolase inhibitor | 48 h | 增强 |
| Siller-Pérez et al., | 20 | 11 | 大鼠 | inhibitory avoidance | WIN55, 212-2 into the dorsal striatum | 48 h | 增强 |
| Cahill et al., | 25 | 23 | 人类 | slides of varying emotional content | cold pressor stress | 1 wk | 增强 |
| van Marle et al., | 20 | 19 | 人类 | negative and neutral pictures | administered hydrocortisone during sleep | 1 d | 增强 |
| Borota et al., | 35 | 38 | 人类 | studied images of objects | 200 mg of caffeine | 24 h | 增强 |
| McCullough et al., | 23 | 24 | 人类 | emotional and neutral pictures | cold-pressor test | 24 h | 倒U |
| Krenz et al., | 52 | 52 | 人类 | negative and neutral pictures | α2-adrenoceptor antagonist yohimbine | 1 d, 28 d | 增强 |
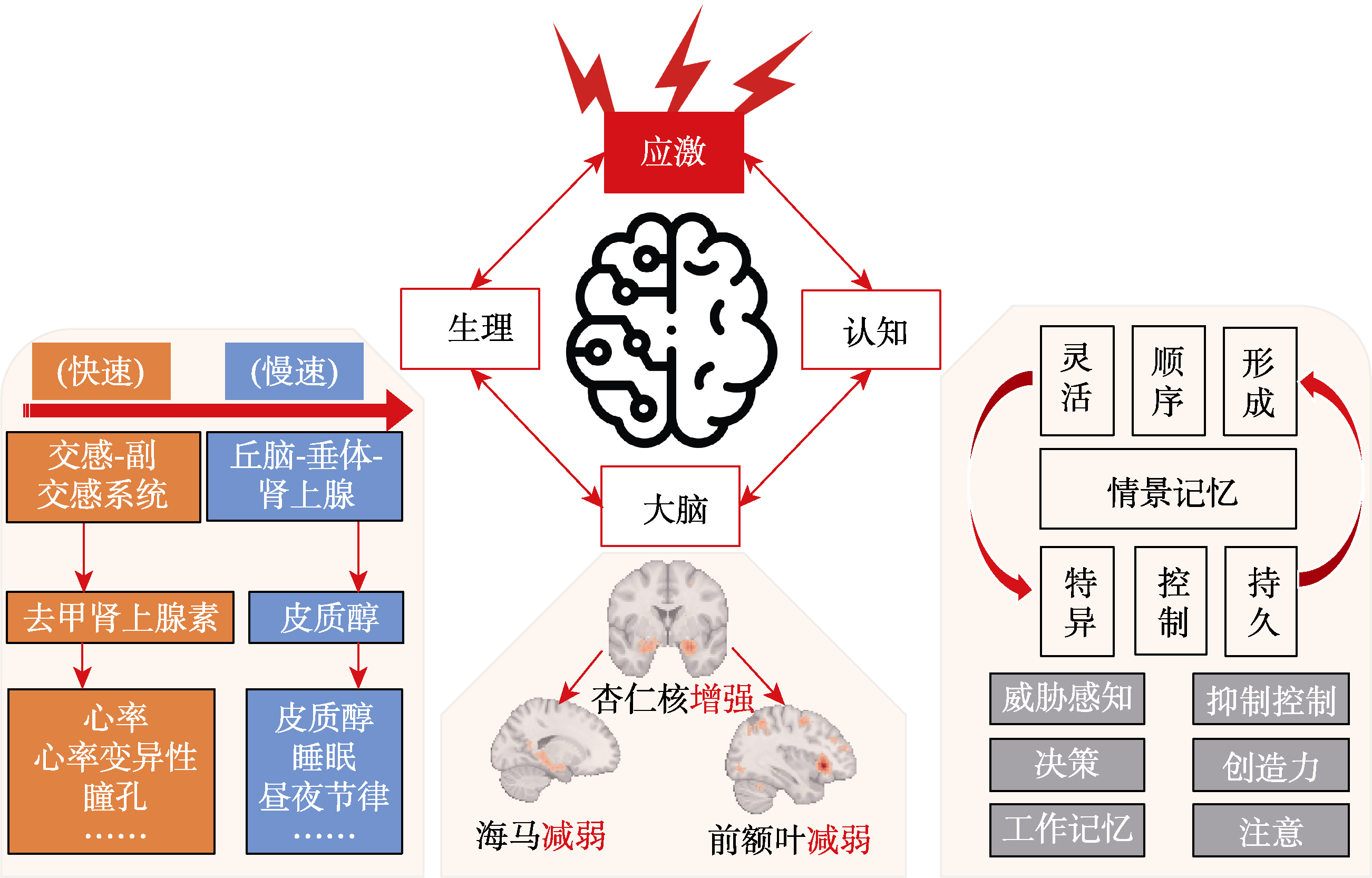
图1 应激下生理−大脑−认知响应的整合模型。生理层面上, 应激反应开始于交感−副交感系统快速响应, 系统响应导致去甲肾上腺素分泌, 带动心率和瞳孔的相关变化; 而丘脑−垂体−肾上腺系统随后响应, 皮质醇分泌, 反映在唾液和头发皮质醇的相关变化和睡眠改变。大脑层面上, 应激主要影响前额叶−海马−杏仁核环路活动: 应激会增强杏仁核活动, 同时减弱前额叶−海马环路的活动强度和信息交互。认知层面上, 应激会影响多个认知功能(如: 决策, 注意, 创造力), 本研究主要关注其对情景记忆的影响。以往研究发现应激会影响情景记忆的不同属性(如: 形成效率、持久性、灵活性、顺序性等), 本课题拟将神经重放的不同特征与情景记忆的各个方面关联起来。
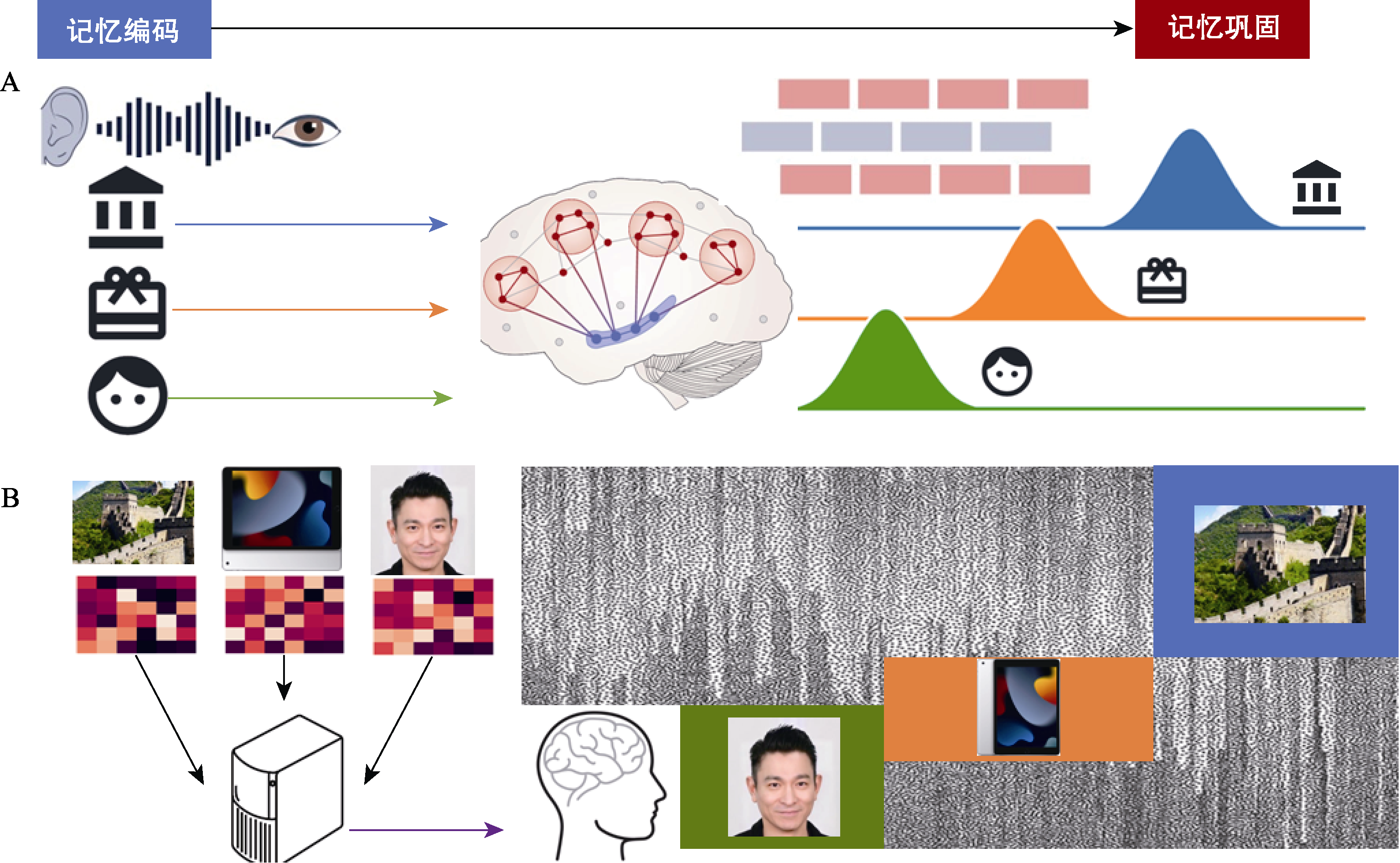
图3 结合神经解码和计算模型刻画人类记忆巩固期的神经重放。(A)记忆编码后, 多个记忆信息会进入巩固阶段。此时, 海马和广泛的皮层区域会发生信息交互, 记忆信息会在大脑中以一定顺序(顺序或倒序)重新出现, 这种现象称之为神经重放(Neural Replay)。神经重放被认为是记忆巩固(Memory Consolidation)的关键神经过程。(B)为了在实验数据中捕捉到人类神经重放, 我们首先需要建立神经模式与外界刺激(通常是视觉, 如地点, 物品, 人脸)的关联, 即训练出机器学习算法可以解码记忆内容。然后, 通过把机器学习算法应用到记忆巩固期的自发神经活动, 可以获得在特点时间点, 各类外界刺激在大脑中表征的概率信息。最后, 通过进一步建立不同记忆出现时间点的顺序模型(Liu, Dolan et al., 2021; Liu, Nour et al., 2022), 从而对神经重放进行量化。
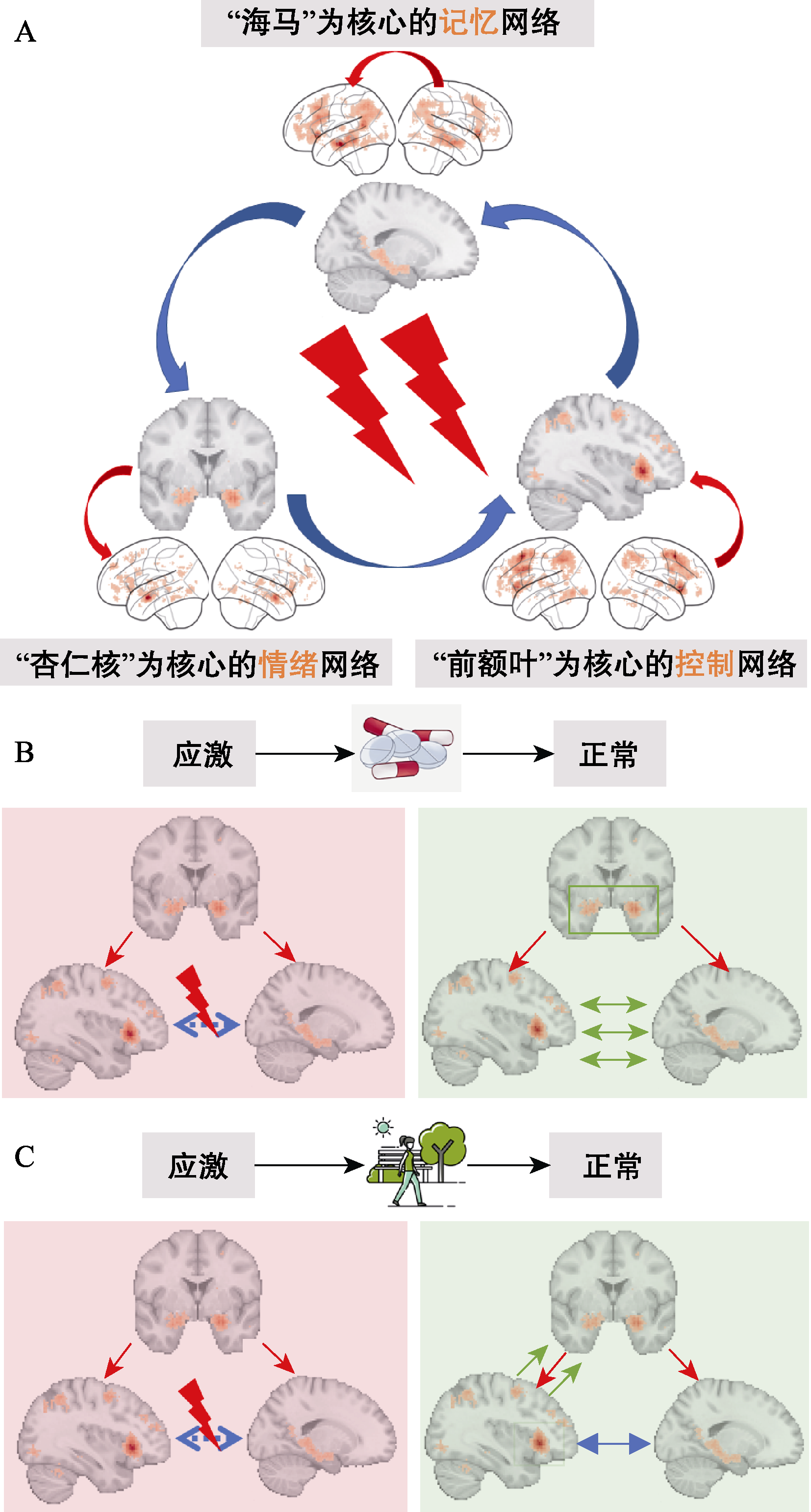
图4 应激下脑网络交互模式及其调控机制。(A)应激下的记忆功能主要涉及三大网络: 以海马为核心的记忆网络, 以杏仁核为核心的情绪网络, 和以前额叶为核心的控制网络。(B)神经内分泌策略调控应激响应的可能神经机制在于: 降低杏仁核的神经活动, 以恢复前额叶−海马环路正常的信息交互。(C)环境策略调控应激响应的可能神经机制的可能机制在于: 增强前额叶活动和前额叶对杏仁核的控制, 以恢复前额叶−海马环路的正常活动。
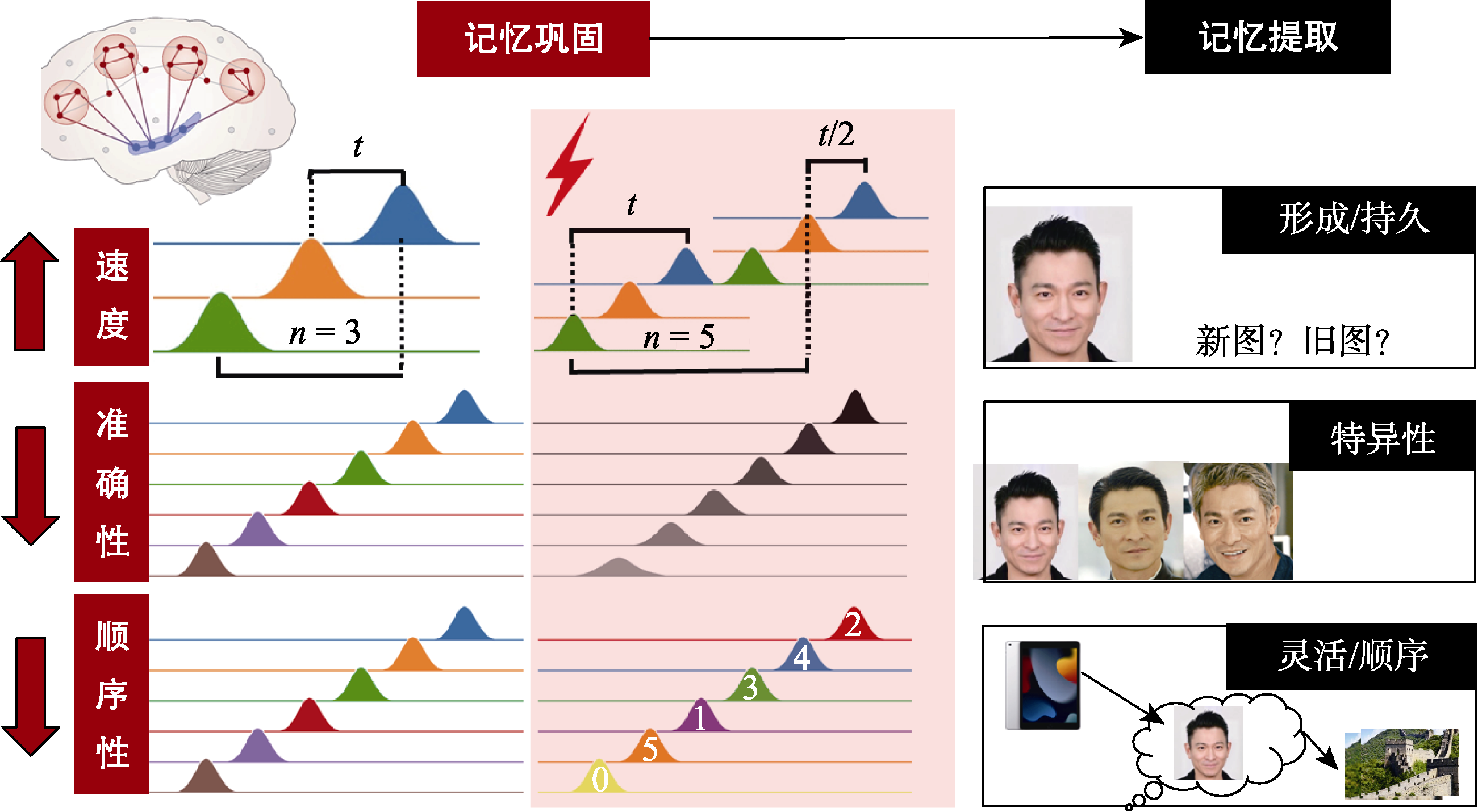
图5 应激对人类记忆巩固的“双刃剑”效应假说。通过采集记忆巩固时的高时间分辨率的脑电图或脑磁图信号, 项目将利用计算神经科学工具分别量化神经重放的速度, 准确性和顺序性。理论假设为: 应激并非只是简单地增强(或削弱)记忆巩固, 而是加速神经重放的速度, 但同时会扰乱其准确性和顺序性。为了探究应激状态下神经重放指标变化的行为后果, 项目将在记忆提取阶段, 采用不同范式对记忆的持久性、特异性和灵活性三方面进行测试。
| [1] |
Ambrose, R. E., Pfeiffer, B. E., & Foster, D. J. (2016). Reverse replay of hippocampal place cells is uniquely modulated by changing reward. Neuron, 91(5), 1124-1136. https://doi.org/10.1016/j.neuron.2016.07.047
doi: S0896-6273(16)30463-9 URL pmid: 27568518 |
| [2] |
Antony, J. W., Ferreira, C. S., Norman, K. A., & Wimber, M. (2017). Retrieval as a fast route to memory consolidation. Trends in Cognitive Sciences, 21(8), 573-576. https://doi.org/10.1016/j.tics.2017.05.001
doi: S1364-6613(17)30099-2 URL pmid: 28583416 |
| [3] | Armario, A., Escorihuela, R. M., & Nadal, R. (2008). Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neuroscience & Biobehavioral Reviews, 32(6), 1121-1135. https://doi.org/10.1016/j.neubiorev.2008.04.003 |
| [4] |
Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410-422. https://doi.org/10.1038/nrn2648
doi: 10.1038/nrn2648 URL pmid: 19455173 |
| [5] |
Barsegyan, A., McGaugh, J. L., & Roozendaal, B. (2014). Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Frontiers in Behavioral Neuroscience, 8, 160. https://doi.org/10.3389/fnbeh.2014.00160
doi: 10.3389/fnbeh.2014.00160 URL pmid: 24847228 |
| [6] | Bass, D. I., Partain, K. N., & Manns, J. R. (2012). Event- specific enhancement of memory via brief electrical stimulation to the basolateral complex of the amygdala in rats. Behavioral Neuroscience, 126(1), 204-208. https://doi.org/10.1037/a0026462 |
| [7] |
Berman, M. G., Kardan, O., Kotabe, H. P., Nusbaum, H. C., & London, S. E. (2019). The promise of environmental neuroscience. Nature Human Behaviour, 3(5), 414-417. https://doi.org/10.1038/s41562-019-0577-7
doi: 10.1038/s41562-019-0577-7 URL pmid: 31089299 |
| [8] | Berto, R. (2014). The role of nature in coping with psycho- physiological stress:A literature review on restorativeness. In Behavioral Sciences (Vol. 4, Issue 4, pp. 394-409). MDPI Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/bs4040394 |
| [9] | Bogdanov, M., & Schwabe, L. (2016). Transcranial stimulation of the dorsolateral prefrontal cortex prevents stress- induced working memory deficits. The Journal of Neuroscience, 36(4), 1429-1437. https://doi.org/10.1523/JNEUROSCI.3687-15.2016 |
| [10] |
Borota, D., Murray, E., Keceli, G., Chang, A., Watabe, J. M., Ly, M., Toscano, J. P., & Yassa, M. A. (2014). Post-study caffeine administration enhances memory consolidation in humans. Nature Neuroscience, 17(2), 201-203. https://doi. org/10.1038/nn.3623
doi: 10.1038/nn.3623 pmid: 24413697 |
| [11] |
Buchanan, T. W., & Lovallo, W. R. (2001). Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology, 26(3), 307-317. https://doi.org/10.1016/S0306-4530(00)00058-5
URL pmid: 11166493 |
| [12] | Cahill, L., Babinsky, R., Markowitsch, H. J., & McGaugh, J. L. (1995). The amygdala and emotional memory. Nature, 377(6547), 295-296. https://doi.org/10.1038/377295a0 |
| [13] | Cahill, L., Gorski, L., & Le, K. (2003). Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory, 10(4), 270-274. https://doi.org/10.1101/lm.62403 |
| [14] | Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., … McGaugh, J. L. (1996). Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences, 93(15), 8016-8021. https://doi.org/10.1073/pnas.93.15.8016 |
| [15] | Cahill, L., Prins, B., Weber, M., & McGaugh, J. L. (1994). β-adrenergic activation and memory for emotional events. Nature, 371(6499), 702-704. https://doi.org/10.1038/371702a0 |
| [16] |
Campolongo, P., Roozendaal, B., Trezza, V., Hauer, D., Schelling, G., McGaugh, J. L., & Cuomo, V. (2009). Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proceedings of the National Academy of Sciences of the United States of America, 106(12), 4888-4893. https://doi.org/10.1073/pnas.0900835106
doi: 10.1073/pnas.0900835106 URL pmid: 19255436 |
| [17] |
Carr, M. F., Jadhav, S. P., & Frank, L. M. (2011). Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nature Neuroscience, 14(2), 147-153. https://doi.org/10.1038/nn.2732
doi: 10.1038/nn.2732 URL pmid: 21270783 |
| [18] | Chang, J., Hu, J., Li, C.-S. R., & Yu, R. (2020). Neural correlates of enhanced response inhibition in the aftermath of stress. NeuroImage, 204, 116212. https://doi.org/10.1016/j.neuroimage.2019.116212 |
| [19] | Chang, J., & Yu, R. (2019). Hippocampal connectivity in the aftermath of acute social stress. Neurobiology of Stress, 11, 100195. https://doi.org/10.1016/j.ynstr.2019.100195 |
| [20] |
Cohen, J. D., Daw, N., Engelhardt, B., Hasson, U., Li, K., Niv, Y., … Willke, T. L. (2017). Computational approaches to fMRI analysis. Nature Neuroscience, 20(3), 304-313. https://doi.org/10.1038/nn.4499
doi: 10.1038/nn.4499 URL pmid: 28230848 |
| [21] | Cousijn, H., Rijpkema, M., Qin, S., van Marle, H. J. F., Franke, B., Hermans, E. J., … Fernández, G. (2010). Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences, 107(21), 9867-9872. https://doi.org/10.1073/pnas.1003514107 |
| [22] |
Davidson, T. J., Kloosterman, F., & Wilson, M. A. (2009). Hippocampal replay of extended experience. Neuron, 63(4), 497-507. https://doi.org/10.1016/j.neuron.2009.07.027
doi: 10.1016/j.neuron.2009.07.027 URL pmid: 19709631 |
| [23] |
de Kloet, E. R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463-475. https://doi.org/10.1038/nrn1683
doi: 10.1038/nrn1683 URL pmid: 15891777 |
| [24] | de Quervain, D. J.-F., Aerni, A., & Roozendaal, B. (2007). Preventive effect of β-Adrenoceptor blockade on glucocorticoid- induced memory retrieval deficits. American Journal of Psychiatry, 164(6), 967-969. https://doi.org/10.1176/ajp.2007.164.6.967 |
| [25] | de Quervain, D. J.-F., Roozendaal, B., & McGaugh, J. L. (1998). Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature, 394(6695), 787-790. https://doi.org/10.1038/29542 |
| [26] |
Deisseroth, K. (2011). Optogenetics. Nature Methods, 8(1), 26-29. https://doi.org/10.1038/nmeth.f.324
doi: 10.1038/nmeth.f.324 URL pmid: 21191368 |
| [27] | Diamond, D. M., & Rose, G. M. (1994). Stress impairs LTP and hippocampal-dependent memory. Annals of the New York Academy of Sciences, 746, 411-414. https://doi.org/10.1111/j.1749-6632.1994.tb39271.x |
| [28] |
Dolcos, F., LaBar, K. S., & Cabeza, R. (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron, 42(5), 855-863. https://doi.org/10.1016/S0896-6273(04)00289-2
doi: 10.1016/s0896-6273(04)00289-2 URL pmid: 15182723 |
| [29] |
Dornelles, A., de Lima, M. N. M., Grazziotin, M., Presti- Torres, J., Garcia, V. A., Scalco, F. S., Roesler, R., & Schröder, N. (2007). Adrenergic enhancement of consolidation of object recognition memory. Neurobiology of Learning and Memory, 88(1), 137-142. https://doi.org/10.1016/j.nlm.2007.01.005
URL pmid: 17368053 |
| [30] | Duan, H., Wang, X., Hu, W., & Kounios, J. (2020). Effects of acute stress on divergent and convergent problem-solving. Thinking & Reasoning, 26(1), 68-86. https://doi.org/10.1080/13546783.2019.1572539 |
| [31] | Duan, H., Wang, X., Wang, Z., Xue, W., Kan, Y., Hu, W., & Zhang, F. (2019). Acute stress shapes creative cognition in trait anxiety. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.01517 |
| [32] |
Dupret, D., O’Neill, J., Pleydell-Bouverie, B., & Csicsvari, J. (2010). The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nature Neuroscience, 13(8), 995-1002. https://doi.org/10.1038/nn.2599
doi: 10.1038/nn.2599 URL pmid: 20639874 |
| [33] | Ego-Stengel, V., & Wilson, M. A. (2009). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus, https://doi.org/10.1002/hipo.20707 |
| [34] |
Fernández, G., Effern, A., Grunwald, T., Pezer, N., Lehnertz, K., Dümpelmann, M., … Elger, C. E. (1999). Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science, 285(5433), 1582-1585. https://doi.org/10.1126/science.285.5433.1582
doi: 10.1126/science.285.5433.1582 URL pmid: 10477525 |
| [35] |
Fernández-Ruiz, A., Oliva, A., Fermino de Oliveira, E., Rocha-Almeida, F., Tingley, D., & Buzsáki, G. (2019). Long-duration hippocampal sharp wave ripples improve memory. Science, 364(6445), 1082-1086. https://doi.org/10.1126/science.aax0758
doi: 10.1126/science.aax0758 URL pmid: 31197012 |
| [36] | Ferreira, C. S., Charest, I., & Wimber, M. (2019). Retrieval aids the creation of a generalised memory trace and strengthens episode-unique information. NeuroImage, 201, 115996. https://doi.org/10.1016/j.neuroimage.2019.07.009 |
| [37] | Foster, D. J., & Wilson, M. A. (2006). Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature, 440(7084), 680-683. https://doi. org/10.1038/nature04587 |
| [38] |
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700-711. https://doi.org/10.1038/nrn2201
doi: 10.1038/nrn2201 URL pmid: 17704812 |
| [39] |
Frankland, P. W., & Bontempi, B. (2005). The organization of recent and remote memories. Nature Reviews Neuroscience, 6(2), 119-130. https://doi.org/10.1038/nrn1607
doi: 10.1038/nrn1607 URL pmid: 15685217 |
| [40] |
Frankland, P. W., Josselyn, S. A., & Köhler, S. (2019). The neurobiological foundation of memory retrieval. Nature Neuroscience, 22(10), 1576-1585. https://doi.org/10.1038/s41593-019-0493-1
doi: 10.1038/s41593-019-0493-1 URL pmid: 31551594 |
| [41] | Gagnon, S. A., Waskom, M. L., Brown, T. I., & Wagner, A. D. (2019). Stress impairs episodic retrieval by disrupting hippocampal and cortical mechanisms of remembering. Cerebral Cortex, 29(7), 2947-2964. https://doi.org/10.1093/cercor/bhy162 |
| [42] |
Gärtner, M., Rohde-Liebenau, L., Grimm, S., & Bajbouj, M. (2014). Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology, 43, 105-113. https://doi.org/10.1016/j.psyneuen.2014.02.009
doi: 10.1016/j.psyneuen.2014.02.009 URL pmid: 24703176 |
| [43] | Guerra-Carrillo, B., Mackey, A. P., & Bunge, S. A. (2014). Resting-state fMRI. The Neuroscientist, 20(5), 522-533. https://doi.org/10.1177/1073858414524442 |
| [44] |
Hermans, E. J., Henckens, M. J. A. G., Joëls, M., & Fernández, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37(6), 304-314. https://doi.org/10.1016/j.tins.2014.03.006
doi: 10.1016/j.tins.2014.03.006 URL pmid: 24766931 |
| [45] |
Hermans, E. J., van Marle, H. J. F., Ossewaarde, L., Henckens, M. J. A. G., Qin, S., van Kesteren, M. T. R., … Fernández, G. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science, 334(6059), 1151-1153. https://doi.org/10.1126/science.1209603
doi: 10.1126/science.1209603 URL pmid: 22116887 |
| [46] | Hu, X., Cheng, L. Y., Chiu, M. H., & Paller, K. A. (2020). Promoting memory consolidation during sleep: A meta- analysis of targeted memory reactivation. Psychological Bulletin, 146(3), 218-244. https://doi.org/10.1037/bul0000223 |
| [47] | Inman, C. S., Manns, J. R., Bijanki, K. R., Bass, D. I., Hamann, S., Drane, D. L., … Willie, J. T. (2018). Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proceedings of the National Academy of Sciences, 115(1), 98-103. https://doi.org/10.1073/pnas.1714058114 |
| [48] |
Ji, D., & Wilson, M. A. (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience, 10(1), 100-107. https://doi.org/10.1038/nn1825
doi: 10.1038/nn1825 URL pmid: 17173043 |
| [49] |
Karlsson, M. P., & Frank, L. M. (2009). Awake replay of remote experiences in the hippocampus. Nature Neuroscience, 12(7), 913-918. https://doi.org/10.1038/nn.2344
doi: 10.1038/nn.2344 URL pmid: 19525943 |
| [50] |
Klinzing, J. G., Niethard, N., & Born, J. (2019). Mechanisms of systems memory consolidation during sleep. Nature Neuroscience, 22(10), 1598-1610. https://doi.org/10.1038/s41593-019-0467-3
doi: 10.1038/s41593-019-0467-3 URL pmid: 31451802 |
| [51] |
Krenz, V., Sommer, T., Alink, A., Roozendaal, B., & Schwabe, L. (2021). Noradrenergic arousal after encoding reverses the course of systems consolidation in humans. Nature Communications, 12(1), 6054. https://doi.org/10.1038/s41467-021-26250-7
doi: 10.1038/s41467-021-26250-7 URL pmid: 34663784 |
| [52] |
Kurth-Nelson, Z., Behrens, T., Wayne, G., Miller, K., Luettgau, L., Dolan, R., … Schwartenbeck, P. (2023). Replay and compositional computation. Neuron, 111(4), 454-469. https://doi.org/10.1016/j.neuron.2022.12.028
doi: 10.1016/j.neuron.2022.12.028 URL pmid: 36640765 |
| [53] |
LaBar, K. S., & Cabeza, R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7(1), 54-64. https://doi.org/10.1038/nrn1825
doi: 10.1038/nrn1825 URL pmid: 16371950 |
| [54] | Lederbogen, F., Kirsch, P., Haddad, L., Streit, F., Tost, H., Schuch, P., … Meyer-Lindenberg, A. (2011). City living and urban upbringing affect neural social stress processing in humans. Nature, 474(7352), 498-501. https://doi.org/10.1038/nature10190 |
| [55] | Lee, K. O., Mai, K. M., & Park, S. (2023). Green space accessibility helps buffer declined mental health during the COVID-19 pandemic: Evidence from big data in the United Kingdom. Nature Mental Health, 1(2), 124-134. https://doi.org/10.1038/s44220-023-00018-y |
| [56] |
Lindauer, R. J. L., Olff, M., van Meijel, E. P. M., Carlier, I. V. E., & Gersons, B. P. R. (2006). Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biological Psychiatry, 59(2), 171-177. https://doi.org/10.1016/j.biopsych.2005.06.033
URL pmid: 16154543 |
| [57] | Liu, F., Xu, J., Guo, L., Qin, W., Liang, M., Schumann, G., & Yu, C. (2023). Environmental neuroscience linking exposome to brain structure and function underlying cognition and behavior. Molecular Psychiatry, 28(1), 17-27. https://doi.org/10.1038/s41380-022-01669-6 |
| [58] |
Liu, W., Kohn, N., & Fernández, G. (2019). Intersubject similarity of personality is associated with intersubject similarity of brain connectivity patterns. NeuroImage, 186, 56-69. https://doi.org/10.1016/j.neuroimage.2018.10.062
doi: S1053-8119(18)32034-2 URL pmid: 30389630 |
| [59] | Liu, W., Kohn, N., & Fernández, G. (2021). Dynamic transitions between neural states are associated with flexible task switching during a memory task. Journal of Cognitive Neuroscience, 33(12), 2559-2588. https://doi.org/10.1162/jocn_a_01779 |
| [60] | Liu, W., Shi, Y., Cousins, J. N., Kohn, N., & Fernández, G. (2022). Hippocampal-medial prefrontal event segmentation and integration contribute to episodic memory formation. Cerebral Cortex, 32(5), 949-969. https://doi.org/10.1093/cercor/bhab258 |
| [61] | Liu, Y., Dolan, R. J., Higgins, C., Penagos, H., Woolrich, M. W., Ólafsdóttir, H. F., … Behrens, T. E. (2021). Temporally delayed linear modelling (TDLM) measures replay in both animals and humans. eLife, 10. https://doi.org/10.7554/eLife.66917 |
| [62] |
Liu, Y., Dolan, R. J., Kurth-Nelson, Z., & Behrens, T. E. J. (2019). Human replay spontaneously reorganizes experience. Cell, 178(3), 640-652.e14. https://doi.org/10.1016/j.cell.2019.06.012
doi: S0092-8674(19)30640-3 URL pmid: 31280961 |
| [63] | Liu, Y., Mattar, M. G., Behrens, T. E. J., Daw, N. D., & Dolan, R. J. (2021). Experience replay is associated with efficient nonlocal learning. Science, 372(6544). https://doi.org/10.1126/science.abf1357 |
| [64] |
Liu, Y., Nour, M. M., Schuck, N. W., Behrens, T. E. J., & Dolan, R. J. (2022). Decoding cognition from spontaneous neural activity. Nature Reviews Neuroscience, 23(4), 204-214. https://doi.org/10.1038/s41583-022-00570-z
doi: 10.1038/s41583-022-00570-z URL pmid: 35260845 |
| [65] |
Luo, Y., Fernández, G., Hermans, E., Vogel, S., Zhang, Y., Li, H., & Klumpers, F. (2018). How acute stress may enhance subsequent memory for threat stimuli outside the focus of attention: DLPFC-amygdala decoupling. NeuroImage, 171, 311-322. https://doi.org/10.1016/j.neuroimage.2018.01.010
doi: S1053-8119(18)30012-0 URL pmid: 29329979 |
| [66] |
McCullough, A. M., Ritchey, M., Ranganath, C., & Yonelinas, A. (2015). Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiology of Learning and Memory, 123, 1-10. https://doi.org/10.1016/j.nlm.2015.04.007
doi: 10.1016/j.nlm.2015.04.007 URL pmid: 25930175 |
| [67] |
McCutcheon, R. A., Reis Marques, T., & Howes, O. D. (2020). Schizophrenia—An overview. JAMA Psychiatry, 77(2), 201-210. https://doi.org/10.1001/jamapsychiatry.2019.3360
doi: 10.1001/jamapsychiatry.2019.3360 URL pmid: 31664453 |
| [68] | McGaugh, J. L. (2003). Memory and emotion: The making of lasting memories. Columbia University Press. |
| [69] | McGaugh, J. L. (2018). Emotional arousal regulation of memory consolidation. Current Opinion in Behavioral Sciences, 19, 55-60. https://doi.org/10.1016/j.cobeha.2017.10.003 |
| [70] |
McReynolds, J. R., Anderson, K. M., Donowho, K. M., & McIntyre, C. K. (2014). Noradrenergic actions in the basolateral complex of the amygdala modulate Arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiology of Learning and Memory, 115, 49-57. https://doi.org/10.1016/j.nlm.2014.08.016
doi: 10.1016/j.nlm.2014.08.016 URL pmid: 25196704 |
| [71] |
Morena, M., Roozendaal, B., Trezza, V., Ratano, P., Peloso, A., Hauer, D., Atsak, P., Trabace, L., Cuomo, V., McGaugh, J. L., Schelling, G., & Campolongo, P. (2014). Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proceedings of the National Academy of Sciences of the United States of America, 111(51), 18333-18338. https://doi.org/10.1073/pnas.1420285111
doi: 10.1073/pnas.1420285111 URL pmid: 25489086 |
| [72] | Murayama, K., & Kitagami, S. (2014). Consolidation power of extrinsic rewards: Reward cues enhance long-term memory for irrelevant past events. Journal of Experimental Psychology: General, 143(1), 15-20. https://doi.org/10.1037/a0031992 |
| [73] |
Murayama, K., & Kuhbandner, C. (2011). Money enhances memory consolidation - But only for boring material. Cognition, 119(1), 120-124. https://doi.org/10.1016/j.cognition.2011.01.001
doi: 10.1016/j.cognition.2011.01.001 URL pmid: 21292249 |
| [74] |
Newcomer, J. W., Selke, G., Melson, A. K., Hershey, T., Craft, S., Richards, K., & Alderson, A. L. (1999). Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry, 56(6), 527-533.
doi: 10.1001/archpsyc.56.6.527 pmid: 10359467 |
| [75] | Nguyen, N.D., Lutas, A., Amsalem, O. et al. (2023). Cortical reactivations predict future sensory responses. Natures. https://doi.org/10.1038/s41586-023-06810-1 |
| [76] |
Nour, M. M., Liu, Y., Arumuham, A., Kurth-Nelson, Z., & Dolan, R. J. (2021). Impaired neural replay of inferred relationships in schizophrenia. Cell, 184(16), 4315-4328.e17. https://doi.org/10.1016/j.cell.2021.06.012
doi: 10.1016/j.cell.2021.06.012 URL pmid: 34197734 |
| [77] | Qin, S., Hermans, E. J., van Marle, H. J. F., Luo, J., & Fernández, G. (2009). Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry, 66(1), 25-32. https://doi.org/10.1016/j.biopsych.2009.03.006 |
| [78] |
Rao, R. P., Anilkumar, S., McEwen, B. S., & Chattarji, S. (2012). Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biological Psychiatry, 72(6), 466-475. https://doi.org/10.1016/j.biopsych.2012.04.008
doi: 10.1016/j.biopsych.2012.04.008 URL pmid: 22572034 |
| [79] |
Rasch, B., Büchel, C., Gais, S., & Born, J. (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science, 315(5817), 1426-1429. https://doi. org/10.1126/science.1138581
doi: 10.1126/science.1138581 pmid: 17347444 |
| [80] |
Roozendaal, B., Castello, N. A., Vedana, G., Barsegyan, A., & McGaugh, J. L. (2008). Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory, 90(3), 576-579. https://doi.org/10.1016/j.nlm.2008.06.010
doi: 10.1016/j.nlm.2008.06.010 URL pmid: 18657626 |
| [81] |
Roozendaal, B., Okuda, S., de Quervain, D. J.-F., & McGaugh, J. L. (2006). Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience, 138(3), 901-910. https://doi.org/10.1016/j.neuroscience.2005.07.049
URL pmid: 16310958 |
| [82] | Schuck, N. W., & Niv, Y. (2019a). Sequential replay of nonspatial task states in the human hippocampus. Science, 364(6447). https://doi.org/10.1126/science.aaw5181 |
| [83] | Schuck, N. W., & Niv, Y. (2019b). Sequential replay of nonspatial task states in the human hippocampus. Science, 364(6447). https://doi.org/10.1126/science.aaw5181 |
| [84] | Schwabe, L., Hermans, E. J., Joëls, M., & Roozendaal, B. (2022). Mechanisms of memory under stress. Neuron, 110(9), 1450-1467. https://doi.org/10.1016/j.neuron.2022.02.020 |
| [85] |
Schwabe, L., Römer, S., Richter, S., Dockendorf, S., Bilak, B., & Schächinger, H. (2009). Stress effects on declarative memory retrieval are blocked by a β-adrenoceptor antagonist in humans. Psychoneuroendocrinology, 34(3), 446-454. https://doi.org/10.1016/j.psyneuen.2008.10.009
doi: 10.1016/j.psyneuen.2008.10.009 URL pmid: 19028019 |
| [86] | Sharot, T., & Phelps, E. A. (2004). How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience, 4( 3), 294-306. https://doi.org/10.3758/CABN.4.3.294 |
| [87] |
Siller-Pérez, C., Fuentes-Ibañez, A., Sotelo-Barrera, E. L., Serafín, N., Prado-Alcalá, R. A., Campolongo, P., Roozendaal, B., & Quirarte, G. L. (2019). Glucocorticoid interactions with the dorsal striatal endocannabinoid system in regulating inhibitory avoidance memory. Psychoneuroendocrinology, 99, 97-103. https://doi.org/10.1016/j.psyneuen.2018.08.021
doi: S0306-4530(18)30045-3 URL pmid: 30216767 |
| [88] | Squire, L. R., Genzel, L., Wixted, J. T., & Morris, R. G. (2015). Memory consolidation. Cold Spring Harbor Perspectives in Biology, 7(8), a021766.https://doi.org/ 10.1101/cshperspect.a021766 |
| [89] |
Sudimac, S., Sale, V., & Kühn, S. (2022). How nature nurtures: Amygdala activity decreases as the result of a one-hour walk in nature. Molecular Psychiatry, 27(11), 4446-4452. https://doi.org/10.1038/s41380-022-01720-6
doi: 10.1038/s41380-022-01720-6 URL pmid: 36059042 |
| [90] | Suh, J., Foster, D. J., Davoudi, H., Wilson, M. A., & Tonegawa, S. (2013). Impaired hippocampal ripple- associated replay in a mouse model of schizophrenia. Neuron, 80(2), 484-493. https://doi.org/10.1016/j.neuron.2013.09.014 |
| [91] | Takashima, A., Nieuwenhuis, I. L. C., Jensen, O., Talamini, L. M., Rijpkema, M., & Fernández, G. (2009). Shift from hippocampal to neocortical centered retrieval network with consolidation. The Journal of Neuroscience, 29(32), 10087-10093. https://doi.org/10.1523/JNEUROSCI.0799-09.2009 |
| [92] | Takashima, A., Petersson, K. M., Rutters, F., Tendolkar, I., Jensen, O., Zwarts, M. J., … Fernández, G. (2006). Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proceedings of the National Academy of Sciences, 103(3), 756-761. https://doi.org/10.1073/pnas.0507774103 |
| [93] | Tambini, A., & Davachi, L. (2013). Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proceedings of the National Academy of Sciences, 110(48), 19591-19596. https://doi.org/10.1073/pnas.1308499110 |
| [94] |
Tambini, A., & Davachi, L. (2019). Awake reactivation of prior experiences consolidates memories and biases cognition. Trends in Cognitive Sciences, 23(10), 876-890. https://doi.org/10.1016/j.tics.2019.07.008
doi: S1364-6613(19)30183-4 URL pmid: 31445780 |
| [95] |
Tambini, A., & D’Esposito, M. (2020). Causal contribution of awake post-encoding processes to episodic memory consolidation. Current Biology, 30(18), 3533-3543.e7. https://doi.org/10.1016/j.cub.2020.06.063
doi: S0960-9822(20)30915-5 URL pmid: 32735812 |
| [96] |
Tambini, A., Ketz, N., & Davachi, L. (2010). Enhanced brain correlations during rest are related to memory for recent experiences. Neuron, 65(2), 280-290. https://doi.org/10.1016/j.neuron.2010.01.001
doi: 10.1016/j.neuron.2010.01.001 URL pmid: 20152133 |
| [97] |
Tambini, A., Rimmele, U., Phelps, E. A., & Davachi, L. (2017). Emotional brain states carry over and enhance future memory formation. Nature Neuroscience, 20(2), 271-278. https://doi.org/10.1038/nn.4468
doi: 10.1038/nn.4468 URL pmid: 28024158 |
| [98] | Tost, H., Champagne, F. A., & Meyer-Lindenberg, A. (2015). Environmental influence in the brain, human welfare and mental health. Nature Neuroscience, 18(10), 4121-4131. https://doi.org/10.1038/nn.4108 |
| [99] |
Tse, D., Langston, R. F., Kakeyama, M., Bethus, I., Spooner, P. A., Wood, E. R., … Morris, R. G. M. (2007). Schemas and memory consolidation. Science, 316(5821), 76-82. https://doi.org/10.1126/science.1135935
doi: 10.1126/science.1135935 URL pmid: 17412951 |
| [100] | van Kesteren, M. T. R., Fernández, G., Norris, D. G., & Hermans, E. J. (2010). Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences, 107(16), 7550-7555. https://doi.org/10.1073/pnas.0914892107 |
| [101] |
van Marle, H. J. F., Hermans, E. J., Qin, S., & Fernández, G. (2009). From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry, 66(7), 649-655. https://doi.org/10.1016/j.biopsych.2009.05.014
doi: 10.1016/j.biopsych.2009.05.014 URL pmid: 19596123 |
| [102] |
van Marle, H. J. F., Hermans, E. J., Qin, S., Overeem, S., & Fernández, G. (2013). The effect of exogenous cortisol during sleep on the behavioral and neural correlates of emotional memory consolidation in humans. Psychoneuroendocrinology, 38(9), 1639-1649. https://doi. org/10.1016/j.psyneuen.2013.01.009
doi: 10.1016/j.psyneuen.2013.01.009 pmid: 23484632 |
| [103] | Wamsley, E. J. (2022). Offline memory consolidation during waking rest. Nature Reviews Psychology, 1(8), 441-453. https://doi.org/10.1038/s44159-022-00072-w |
| [104] | Wilhelm, I., Diekelmann, S., Molzow, I., Ayoub, A., Mölle, M., & Born, J. (2011). Sleep selectively enhances memory expected to be of future relevance. The Journal of Neuroscience, 31(5), 1563-1569. https://doi.org/10.1523/JNEUROSCI.3575-10.2011 |
| [105] |
Wittkuhn, L., & Schuck, N. W. (2021). Dynamics of fMRI patterns reflect sub-second activation sequences and reveal replay in human visual cortex. Nature Communications, 12(1), 1795. https://doi.org/10.1038/s41467-021-21970-2
doi: 10.1038/s41467-021-21970-2 URL pmid: 33741933 |
| [106] | Wolf, O. T. (2017). Stress and memory retrieval: Mechanisms and consequences. Current Opinion in Behavioral Sciences, 14, 40-46. https://doi.org/10.1016/j.cobeha.2016.12.001 |
| [107] |
Xu, J., Liu, X., Li, Q., Goldblatt, R., Qin, W., Liu, F., … Schumann, G. (2021). Global urbanicity is associated with brain and behaviour in young people. Nature Human Behaviour, 6(2), 279-293. https://doi.org/10.1038/s41562-021-01204-7
doi: 10.1038/s41562-021-01204-7 URL pmid: 34711977 |
| [108] | Ye, Z., Shi, L., Li, A., Chen, C., & Xue, G. (2020). Retrieval practice facilitates memory updating by enhancing and differentiating medial prefrontal cortex representations. eLife, 9. https://doi.org/10.7554/eLife.57023 |
| [109] | Yuen, E. Y., Liu, W., Karatsoreos, I. N., Feng, J., McEwen, B. S., & Yan, Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences, 106(33), 14075-14079. https://doi.org/10.1073/pnas.0906791106 |
| [110] |
Zhuang, L., Wang, J., Xiong, B., Bian, C., Hao, L., Bayley, P. J., & Qin, S. (2021). Rapid neural reorganization during retrieval practice predicts subsequent long-term retention and false memory. Nature Human Behaviour, 6(1), 134-145. https://doi.org/10.1038/s41562-021-01188-4
doi: 10.1038/s41562-021-01188-4 URL pmid: 34621051 |
| [111] |
Zinkin, S., & Miller, A. J. (1967). Recovery of memory after amnesia induced by electroconvulsive shock. Science, 155(3758), 102-104. https://doi.org/10.1126/science.155.3758.102
URL pmid: 6066666 |
| [1] | 唐苏勤, 彭闻捷, 余茵琪, 符仲芳. 丧亲人群网络化心理干预效果的系统综述与元分析[J]. 心理科学进展, 2025, 33(2): 256-273. |
| [2] | 杜夏雨, 赛力古·亚力坤, 袁洁莹, 任志洪. 创伤后应激障碍的网络化心理干预及起效机制[J]. 心理科学进展, 2025, 33(1): 123-135. |
| [3] | 彭芝琳, 郑若颖, 胡晓晴, 张丹丹. 睡眠对婴幼儿学习的记忆巩固作用[J]. 心理科学进展, 2024, 32(2): 287-299. |
| [4] | 周帆, 田昊月, 姜英杰. 记忆快速巩固:基于图式的学习与重复再激活[J]. 心理科学进展, 2024, 32(11): 1854-1871. |
| [5] | 赵荣, 黄钰杰, 克丽比努尔·艾尔肯, 李晶晶, 高军. 不同感觉通道在应激传染中的作用及其神经机制[J]. 心理科学进展, 2023, 31(11): 2142-2154. |
| [6] | 刘笑晗, 陈明隆, 郭静. 机器学习在儿童创伤后应激障碍识别及转归预测中的应用[J]. 心理科学进展, 2022, 30(4): 851-862. |
| [7] | 杨群, 朱兵, 俞奕铭, 张敬敏, 薛孟孟. 应激环境下亲社会性的增加:来自不同类型的亲社会偏好的研究证据[J]. 心理科学进展, 2022, 30(12): 2809-2824. |
| [8] | 郭静, 刘笑晗, 黄宁. 基于长尾效应的儿童创伤后应激障碍转归机制及干预策略[J]. 心理科学进展, 2022, 30(10): 2154-2163. |
| [9] | 郑志伟, 肖凤秋, 邵琦, 赵晓凤, 黄妍, 李娟. 情景记忆成功年老化的神经机制[J]. 心理科学进展, 2022, 30(10): 2254-2268. |
| [10] | 张萦倩, 赵光义, 韩雨薇, 张静怡, 曹成琦, 王力, 张昆林. 创伤后应激障碍的组蛋白修饰机制[J]. 心理科学进展, 2022, 30(1): 98-114. |
| [11] | 武丽丽, 程刚, 张大均. 重复性急性应激对攻击行为的影响及调控机制[J]. 心理科学进展, 2021, 29(8): 1358-1370. |
| [12] | 白玉, 杨海波. 创伤后应激障碍个体对威胁刺激的注意偏向:眼动研究的证据[J]. 心理科学进展, 2021, 29(4): 737-746. |
| [13] | 孙俊芳, 辛自强, 包呼格吉乐图, 刘敏, 岳衡. 幸福感的稳态与跃迁:一个新的整合视角[J]. 心理科学进展, 2021, 29(3): 481-491. |
| [14] | 薛冰, 王雪娇, 马宁, 高军. 催产素调控心理韧性:基于对海马的作用机制[J]. 心理科学进展, 2021, 29(2): 311-322. |
| [15] | 王红波, 关旭旭, 李梓萌. 即刻消退缺损的原因分析及其神经生物学机制[J]. 心理科学进展, 2021, 29(1): 150-159. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||