

CN 11-4766/R
主办:中国科学院心理研究所
出版:科学出版社


心理科学进展 ›› 2022, Vol. 30 ›› Issue (8): 1856-1869.doi: 10.3724/SP.J.1042.2022.01856 cstr: 32111.14.2022.01856
收稿日期:2021-10-09
出版日期:2022-08-15
发布日期:2022-06-23
基金资助:
ZHOU Zhenyou1, KONG Li1,2( ), CHAN Raymond3(
), CHAN Raymond3( )
)
Received:2021-10-09
Online:2022-08-15
Published:2022-06-23
摘要:
微生物-肠-脑轴假设在精神分裂症发病机制中的研究受到越来越多的关注。以往研究初步考察了肠道微生物的构成与精神分裂症患者脑影像和临床表征之间的联系, 但具体的作用路径尚不明确。当前研究通过总结最新研究进展, 并在此基础上提出肠道微生物影响精神分裂症患者大脑结构和功能的机制假设。相关内容对于进一步阐明精神分裂症的病理机制, 为将肠道微生物纳入精神分裂症的评估与干预提供理论基础。
中图分类号:
周振友, 孔丽, 陈楚侨. (2022). 精神分裂症肠道微生物与脑影像和临床表征的关系. 心理科学进展 , 30(8), 1856-1869.
ZHOU Zhenyou, KONG Li, CHAN Raymond. (2022). The relationship between gut microbiota and brain imaging and clinical manifestation in schizophrenia. Advances in Psychological Science, 30(8), 1856-1869.
| 研究者 | SCZ组/HC组 | 相对丰度 | 多样性 |
|---|---|---|---|
| Shen et al., | 64/53 | 增加: 门水平:变形菌 属水平:琥珀酰菌、巨球孢菌、柯林塞拉菌、梭状芽孢杆菌、克雷伯氏菌和甲烷菌 减少: 属水平:布拉氏菌、粪球菌、玫瑰菌 | |
| Schwarz et al., | 28/16 | 增加: 科水平:乳杆菌科、Halothiobacillaceae、布鲁氏菌科和微球菌科 属水平:乳杆菌属、Tropheryma、Halothiobacillus、Saccharophagus、Ochrobactrum, Deferribacter和Halorubrum 减少: 科水平:韦氏球菌科 属水平:Anabaena、Nitrosospira和Gallionella | |
| Nguyen et al., | 25/25 | 增加: 属水平:厌氧球菌属 减少: 门水平:变形菌门 属水平:流感嗜血杆菌属、萨特氏菌属和梭菌属 | 精神分裂症组与健康对照组的肠道菌群α多样性之间无显著差异。 |
| Xu et al., | 84/84 | 增加: 门水平:放线菌门 目水平:放线菌目和鞘氨醇目 科水平:鞘氨醇科 属水平:埃格氏菌属和巨球孢菌属, 粘杆菌属, 青春双歧杆菌, 产气荚膜梭菌 减少: 目水平:红串珠目 科水平:产碱杆菌科, 肠球菌科, 明串珠科, 红串珠科 属水平:肠球菌属 | 精神分裂症患者组肠道菌群多样性显著低于对照组 |
| Li et al., | 82/80 | 增加: 门水平:放线菌 属水平:柯林塞拉氏菌、乳杆菌、琥珀杆菌、莫格巴杆菌、棒状杆菌、未定义乳球菌和未定义真细菌 减少: 门水平:厚壁菌门 属水平:Adlercreutzia、Anaerostipes、乳球菌和粪链球菌 | α多样性在两组之间没有显著差异, 但是β多样性表明两组之间的微生物组组成存在显着的群落水平分离 |
| Manchia et al., | 38/20 | 缺失: 门水平:蓝细菌门 科水平:巴氏杆菌科, cytophagaceae和morganellaceae, 属水平:醋酸杆菌属、嗜血杆菌属、Turicibacter, Obesumbacterium等 种水平:马链球菌种、粪球菌种、血链球菌种等 | 精神分裂症组的α多样性的Shannon指数显著低于健康对照组。 |
| 赵星梅 et al., | 16/18 | 增加: 门水平:放线菌门 属水平:双歧杆菌属、普雷沃菌属和巨单胞菌属 减少: 门水平:拟杆菌门和软壁菌门 属水平:拟杆菌属和柔嫩梭菌属 | α多样性的Ace指数、Chao指数、Shannon指数患者组显著低于健康组, Simpson指数明显高于健康对照组 |
表1 精神分裂症患者与正常人肠道微生物构成差异性的研究结果总结
| 研究者 | SCZ组/HC组 | 相对丰度 | 多样性 |
|---|---|---|---|
| Shen et al., | 64/53 | 增加: 门水平:变形菌 属水平:琥珀酰菌、巨球孢菌、柯林塞拉菌、梭状芽孢杆菌、克雷伯氏菌和甲烷菌 减少: 属水平:布拉氏菌、粪球菌、玫瑰菌 | |
| Schwarz et al., | 28/16 | 增加: 科水平:乳杆菌科、Halothiobacillaceae、布鲁氏菌科和微球菌科 属水平:乳杆菌属、Tropheryma、Halothiobacillus、Saccharophagus、Ochrobactrum, Deferribacter和Halorubrum 减少: 科水平:韦氏球菌科 属水平:Anabaena、Nitrosospira和Gallionella | |
| Nguyen et al., | 25/25 | 增加: 属水平:厌氧球菌属 减少: 门水平:变形菌门 属水平:流感嗜血杆菌属、萨特氏菌属和梭菌属 | 精神分裂症组与健康对照组的肠道菌群α多样性之间无显著差异。 |
| Xu et al., | 84/84 | 增加: 门水平:放线菌门 目水平:放线菌目和鞘氨醇目 科水平:鞘氨醇科 属水平:埃格氏菌属和巨球孢菌属, 粘杆菌属, 青春双歧杆菌, 产气荚膜梭菌 减少: 目水平:红串珠目 科水平:产碱杆菌科, 肠球菌科, 明串珠科, 红串珠科 属水平:肠球菌属 | 精神分裂症患者组肠道菌群多样性显著低于对照组 |
| Li et al., | 82/80 | 增加: 门水平:放线菌 属水平:柯林塞拉氏菌、乳杆菌、琥珀杆菌、莫格巴杆菌、棒状杆菌、未定义乳球菌和未定义真细菌 减少: 门水平:厚壁菌门 属水平:Adlercreutzia、Anaerostipes、乳球菌和粪链球菌 | α多样性在两组之间没有显著差异, 但是β多样性表明两组之间的微生物组组成存在显着的群落水平分离 |
| Manchia et al., | 38/20 | 缺失: 门水平:蓝细菌门 科水平:巴氏杆菌科, cytophagaceae和morganellaceae, 属水平:醋酸杆菌属、嗜血杆菌属、Turicibacter, Obesumbacterium等 种水平:马链球菌种、粪球菌种、血链球菌种等 | 精神分裂症组的α多样性的Shannon指数显著低于健康对照组。 |
| 赵星梅 et al., | 16/18 | 增加: 门水平:放线菌门 属水平:双歧杆菌属、普雷沃菌属和巨单胞菌属 减少: 门水平:拟杆菌门和软壁菌门 属水平:拟杆菌属和柔嫩梭菌属 | α多样性的Ace指数、Chao指数、Shannon指数患者组显著低于健康组, Simpson指数明显高于健康对照组 |
| 研究对象 | 干预方式 | 测量方法 | 结果 | 参考 | |||
|---|---|---|---|---|---|---|---|
| 神经影像结果 | GM结果 | 相关关系 | 其他 | ||||
| 住院精神分裂症患者(SCZ)、健康对照组(HCs) | 无干预(住院精神分裂症患者21人, 健康对照组30人) | 功能磁共振扫描SIEMENS3.0T Primage (粪便样本), PANSS评估 | SCZ的ALFF指数的在双侧枕叶、后顶叶显著下降, 双内侧前额叶、外侧前额叶、内侧颞叶显著上升, 颞上回、双侧枕叶的ReHo值显著下降, 内侧前额叶及外侧前额叶的ReHo值显著上升。 | 与HCs组相比, SCZ组的的放线菌门及其下属的红蝽菌纲等菌群丰度上升, 变形菌门下属的海洋螺菌目等丰度显著上升。 | 基于ALFF与菌群的相关性分析发现:放线菌门与外侧前额叶、右侧额中回显著正相关; Acidminococcaccac的相对丰度与枕叶的ALFF值成显著正相关, 而与颞叶呈负相关。 | SCZ患者肠道菌群中红蝽菌纲相对丰度升高可能是精神分裂症的一个诱因(阴性症状、认知受损) | 吴位东, |
| 首发精神分裂症(FSCZ)、长期用药精神分裂症(TSCZ)、健康对照(HCs) | 无干预(首发40人、长期用药85人、健康对照69人) | 磁共振结构成像、GM (粪便样本) | SCZ患者与HCs之间总灰质体积无显著差异, SCZ患者的右侧额中回的灰质体积显著增加。 | 科水平上:与HCs组相比, FSCZ患者克里斯滕森科、肠杆菌科和骆驼蓬科丰度上升, 巴斯德氏菌科、旅游杆菌科、消化链球菌科、韦氏菌科、琥珀酰菌科的丰度下降。 | 偏相关分析发现放线杆菌属和韦氏菌科的相对丰度与FSCZ患者的右侧额中回的灰质体积呈正相关 | FSCZ和TSCZ患者的肠道生物的改变是SCZ固有的特征, 与抗精神病药物治疗没有特别的相关性。 | Ma et al., |
| 精神分裂症患者(SCZ), 健康对照组(HCs) | 无干预(SCZ患者38人, HCs38人) | 磁共振结构成像、GM (粪便样本) | SCZ患者和HCs在16个脑区的灰质体积存在显著差异, SCZ患者和HCs患者在34个脑区的ReHo值存在显著差异, 只有一个脑区表现出显著的组间ALFF差异。与NCs相比, SZ患者某些脑区的灰质体积降低, 包括双侧岛叶、额叶和颞叶区域。 | 两组之间在α多样性的任何水平上都没有显著差异。与HC组比较, SCZ组乳球菌和玫瑰球菌的相对丰度显著下降, SCZ组的Veillonella相对丰度显著上升, | SCZ患者肠道菌群的α多样性均匀度和Shannon指数与大脑某些区域的灰质体积和ReHo值呈正相关; SCZ患者中的右侧颞上回、左侧楔叶和右侧颞中回的ReHo值与蔷薇属的相对丰度呈负相关。 | Li et al., | |
表2 精神分裂症患者肠道微生物与脑影像相关性研究结果总结
| 研究对象 | 干预方式 | 测量方法 | 结果 | 参考 | |||
|---|---|---|---|---|---|---|---|
| 神经影像结果 | GM结果 | 相关关系 | 其他 | ||||
| 住院精神分裂症患者(SCZ)、健康对照组(HCs) | 无干预(住院精神分裂症患者21人, 健康对照组30人) | 功能磁共振扫描SIEMENS3.0T Primage (粪便样本), PANSS评估 | SCZ的ALFF指数的在双侧枕叶、后顶叶显著下降, 双内侧前额叶、外侧前额叶、内侧颞叶显著上升, 颞上回、双侧枕叶的ReHo值显著下降, 内侧前额叶及外侧前额叶的ReHo值显著上升。 | 与HCs组相比, SCZ组的的放线菌门及其下属的红蝽菌纲等菌群丰度上升, 变形菌门下属的海洋螺菌目等丰度显著上升。 | 基于ALFF与菌群的相关性分析发现:放线菌门与外侧前额叶、右侧额中回显著正相关; Acidminococcaccac的相对丰度与枕叶的ALFF值成显著正相关, 而与颞叶呈负相关。 | SCZ患者肠道菌群中红蝽菌纲相对丰度升高可能是精神分裂症的一个诱因(阴性症状、认知受损) | 吴位东, |
| 首发精神分裂症(FSCZ)、长期用药精神分裂症(TSCZ)、健康对照(HCs) | 无干预(首发40人、长期用药85人、健康对照69人) | 磁共振结构成像、GM (粪便样本) | SCZ患者与HCs之间总灰质体积无显著差异, SCZ患者的右侧额中回的灰质体积显著增加。 | 科水平上:与HCs组相比, FSCZ患者克里斯滕森科、肠杆菌科和骆驼蓬科丰度上升, 巴斯德氏菌科、旅游杆菌科、消化链球菌科、韦氏菌科、琥珀酰菌科的丰度下降。 | 偏相关分析发现放线杆菌属和韦氏菌科的相对丰度与FSCZ患者的右侧额中回的灰质体积呈正相关 | FSCZ和TSCZ患者的肠道生物的改变是SCZ固有的特征, 与抗精神病药物治疗没有特别的相关性。 | Ma et al., |
| 精神分裂症患者(SCZ), 健康对照组(HCs) | 无干预(SCZ患者38人, HCs38人) | 磁共振结构成像、GM (粪便样本) | SCZ患者和HCs在16个脑区的灰质体积存在显著差异, SCZ患者和HCs患者在34个脑区的ReHo值存在显著差异, 只有一个脑区表现出显著的组间ALFF差异。与NCs相比, SZ患者某些脑区的灰质体积降低, 包括双侧岛叶、额叶和颞叶区域。 | 两组之间在α多样性的任何水平上都没有显著差异。与HC组比较, SCZ组乳球菌和玫瑰球菌的相对丰度显著下降, SCZ组的Veillonella相对丰度显著上升, | SCZ患者肠道菌群的α多样性均匀度和Shannon指数与大脑某些区域的灰质体积和ReHo值呈正相关; SCZ患者中的右侧颞上回、左侧楔叶和右侧颞中回的ReHo值与蔷薇属的相对丰度呈负相关。 | Li et al., | |
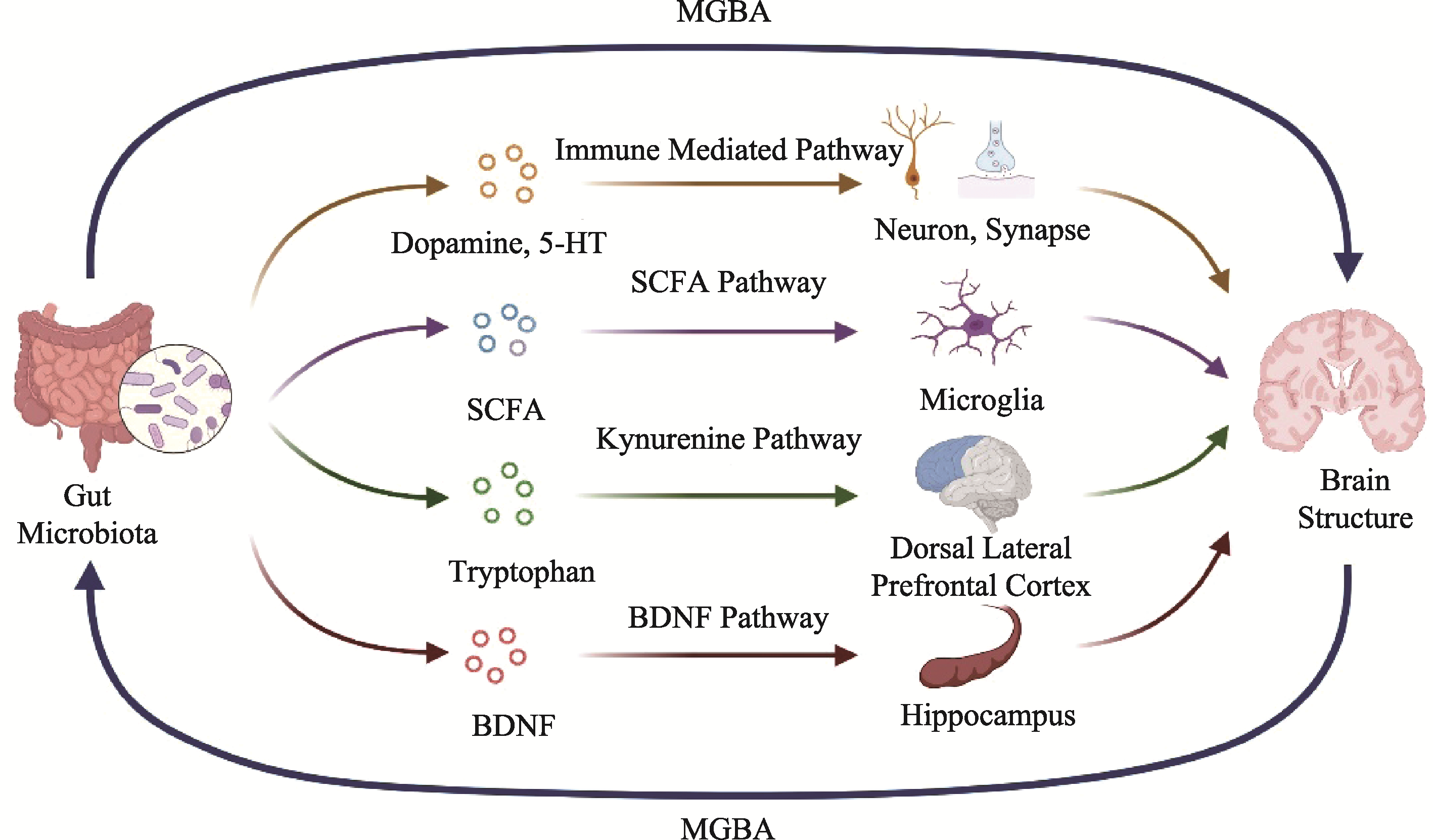
图1 精神分裂症患者肠道微生物作用于大脑结构的机制假设 注:MGBA, 微生物-肠-脑轴; Gut Microbiota, 肠道微生物; Dopamine, 多巴胺; 5-HT, 五羟色胺; Neuron, 神经元; Synapse, 突触; Microglia, 小胶质细胞; Tryptophan, 色氨酸; Kynurenine Pathway, 犬尿氨酸通路; Dorsal Lateral Prefrontal Cortex, 背外侧前额叶; Hippocampus, 海马体; Brain Structure, 大脑结构; SCFA, 短链脂肪酸; BDNF, 脑源性神经营养因子
| [1] | 吴位东. (2019). 精神分裂症患者脑区功能与肠道菌群改变的关联性研究 (硕士学位论文). 内蒙古医科大学. |
| [2] | 赵星梅, 王喜苹, 周火祥, 张红梅, 王秀丽, 罗予, 郭葳. (2019). 基于高通量测序的精神分裂症患者肠道菌群多样性. 中国微生态学杂志, 31(1), 1-7. |
| [3] |
Ahmed A. O., Kramer S., Hofman N., Flynn J., Hansen M., Martin V., Pillai A., & Buckley P. F. (2021). A meta-analysis of brain-derived neurotrophic factor effects on brain volume in schizophrenia: Genotype and serum levels. Neuropsychobiology, 80(5), 411-424.
doi: 10.1159/000514126 URL |
| [4] |
Ait-Belgnaoui A., Durand H., Cartier C., Chaumaz G., Eutamene H., Ferrier L., Houdeau E., Fioramonti J., Bueno L., & Theodorou V. (2012). Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology, 37(11), 1885-1895.
doi: 10.1016/j.psyneuen.2012.03.024 pmid: 22541937 |
| [5] | Bauer K. C., Rees T., & Finlay B. B. (2019). The gut microbiota-brain axis expands neurologic function: A nervous rapport. BioEssays, 41(10), e1800268. https://doi.org/10.1002/bies.201800268 |
| [6] | Bioque M., González-Rodríguez A., Garcia-Rizo C., Cobo J., Monreal J. A., Usall J., Soria V., Labad J., & Group P. (2020). Targeting the microbiome-gut-brain axis for improving cognition in schizophrenia and major mood disorders: A narrative review. Progress in Neuro- Psychopharmacology and Biological Psychiatry, 105, Article 110130. https://doi.org/10.1016/j.pnpbp.2020.110130 |
| [7] |
Cabrera B., Bioque M., Penadés R., González-Pinto A., Parellada M., Bobes J., Lobo A., García-Bueno B., Leza J., & Bernardo M. (2016). Cognition and psychopathology in first-episode psychosis: Are they related to inflammation? Psychological Medicine, 46(10), 2133-2144.
doi: 10.1017/S0033291716000659 pmid: 27055381 |
| [8] |
Cadinu D., Grayson B., Podda G., Harte M. K., Doostdar N., & Neill J. C. (2018). NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology, 142(11), 41-62.
doi: 10.1016/j.neuropharm.2017.11.045 URL |
| [9] |
Cai H. Q., Catts V. S., Webster M. J., Galletly C., Liu D., O’Donnell M., Weickert T. W., & Weickert C. S. (2020). Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Molecular Psychiatry, 25(4), 761-775.
doi: 10.1038/s41380-018-0235-x URL |
| [10] |
Chiappelli J., Pocivavsek A., Nugent K. L., Notarangelo F. M., Kochunov P., Rowland L. M., Schwarcz R., & Hong L. E. (2014). Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry, 71(7), 761-768.
doi: 10.1001/jamapsychiatry.2014.243 pmid: 24806441 |
| [11] |
Cussotto S., Sandhu K. V., Dinan T. G., & Cryan J. F. (2018). The neuroendocrinology of the microbiota-gut- brain axis: A behavioural perspective. Frontiers in Neuroendocrinology, 51(4), 80-101.
doi: 10.1016/j.yfrne.2018.04.002 URL |
| [12] | Dickerson F. B., Stallings C., Origoni A., Katsafanas E., Savage C. L., Schweinfurth L. A., Goga J., Khushalani S., & Yolken R. H. (2014). Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. The Primary Care Companion for CNS Disorders, 16(1), Article 13m01579. https://doi.org/10.4088/PCC.13m01579 |
| [13] | El Aidy S., Ramsteijn A. S., Dini-Andreote F., van Eijk R., Houwing D. J., Salles J. F., & Olivier J. D. (2017). Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress. Frontiers in Cellular Neuroscience, 11, Article 222. https://doi.org/10.3389/fncel.2017.00222 |
| [14] | Ferretti P., Pasolli E., Tett A., Asnicar F., Gorfer V., Fedi S., … Segata N. (2018). Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host & Microbe, 24(1), 133-145.e5. |
| [15] |
Fett A.-K. J., Viechtbauer W., Dominguez M.-D.-G., Penn D. L., van Os J., & Krabbendam L. (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta- analysis. Neuroscience & Biobehavioral Reviews, 35(3), 573-588.
doi: 10.1016/j.neubiorev.2010.07.001 URL |
| [16] |
Foster J. A., & Neufeld K.-A. M. (2013). Gut-brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences, 36(5), 305-312.
doi: 10.1016/j.tins.2013.01.005 pmid: 23384445 |
| [17] |
Gillespie A. L., Samanaite R., Mill J., Egerton A., & MacCabe J. H. (2017). Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? A systematic review. BMC Psychiatry, 17(1), 1-14.
doi: 10.1186/s12888-016-1163-4 URL |
| [18] |
Green M. F., Horan W. P., & Lee J. (2019). Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry, 18(2), 146-161.
doi: 10.1002/wps.20624 URL |
| [19] |
Guo J., Ragland J. D., & Carter C. S. (2019). Memory and cognition in schizophrenia. Molecular Psychiatry, 24(5), 633-642.
doi: 10.1038/s41380-018-0231-1 pmid: 30242229 |
| [20] | Guo L., Xiao P., Zhang X., Yang Y., Yang M., Wang T., Lu H., Tian H., Wang H., & Liu J. (2021). Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food & Function, 12(3), 1156-1175. |
| [21] | Gupta L., & Hoffman K. W. (2021). Exploring the intersection of the microbiome and the developing brain: Impacts on schizophrenia risk. Schizophrenia Research. Advance online publication. https://doi.org/10.1016/j.schres.2021.08.010 |
| [22] |
He Y., Kosciolek T., Tang J., Zhou Y., Li Z., Ma X., Zhu Q., Yuan N., Yuan L., Li C., Jin K., Knight R., Tsuang M. T., & Chen X. (2018). Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. European Psychiatry, 53(5), 37-45.
doi: 10.1016/j.eurpsy.2018.05.011 URL |
| [23] |
Heijtz R. D., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M. L., Forssberg H., & Pettersson S. (2011). Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences, 108(7), 3047-3052.
doi: 10.1073/pnas.1010529108 URL |
| [24] |
Hori H., Noguchi H., Hashimoto R., Nakabayashi T., Omori M., Takahashi S., … Kunugi H. (2006). Antipsychotic medication and cognitive function in schizophrenia. Schizophrenia Research, 86(1-3), 138-146.
doi: 10.1016/j.schres.2006.05.004 URL |
| [25] |
Insel T. R. (2010). Rethinking schizophrenia. Nature, 468(7321), 187-193.
doi: 10.1038/nature09552 URL |
| [26] |
Jadhav K. S., Peterson V. L., Halfon O., Ahern G., Fouhy F., Stanton C., Dinan T. G., Cryan J. F., & Boutrel B. (2018). Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology, 141(8), 249-259.
doi: 10.1016/j.neuropharm.2018.08.026 URL |
| [27] |
Jameson K. G., & Hsiao E. Y. (2018). Linking the gut microbiota to a brain neurotransmitter. Trends in Neurosciences, 41(7), 413-414.
doi: S0166-2236(18)30086-9 pmid: 29933773 |
| [28] |
Jenkins T. A., Nguyen J. C., Polglaze K. E., & Bertrand P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients, 8(1), 56. https://doi.org/10.3390/nu8010056
doi: 10.3390/nu8010056 URL |
| [29] | Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., … Ruan B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48(3), 186-194. |
| [30] |
Kelly D., Conway S., & Aminov R. (2005). Commensal gut bacteria: Mechanisms of immune modulation. Trends in Immunology, 26(6), 326-333.
doi: 10.1016/j.it.2005.04.008 URL |
| [31] |
Kelly J. R., Minuto C., Cryan J. F., Clarke G., & Dinan T. G. (2021). The role of the gut microbiome in the development of schizophrenia. Schizophrenia Research, 234(2), 4-23.
doi: 10.1016/j.schres.2020.02.010 URL |
| [32] |
Kindler J., Lim C. K., Weickert C. S., Boerrigter D., Galletly C., Liu D., … Weickert T. W. (2020). Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Molecular Psychiatry, 25(11), 2860-2872.
doi: 10.1038/s41380-019-0401-9 URL |
| [33] | Li Q., Han Y., Dy A. B. C., & Hagerman R. J. (2017). The gut microbiota and autism spectrum disorders. Frontiers in Cellular Neuroscience, 11, Article 120. https://doi.org/10.3389/fncel.2017.00120 |
| [34] | Li S., Song J., Ke P., Kong L., Lei B., Zhou J., … Wu K. (2021). The gut microbiome is associated with brain structure and function in schizophrenia. Scientific Reports, 11(1), 9743. |
| [35] | Li S., Zhuo M., Huang X., Huang Y., Zhou J., Xiong D., … Wu K. (2020). Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ, 8, Article 9574. https://doi.org/10.7717/peerj.9574 |
| [36] | Lloyd-Price J., Abu-Ali G., & Huttenhower C. (2016). The healthy human microbiome. Genome Medicine, 8(1), 51. |
| [37] |
Lv F., Chen S., Wang L., Jiang R., Tian H., Li J., Yao Y., & Zhuo C. (2017). The role of microbiota in the pathogenesis of schizophrenia and major depressive disorder and the possibility of targeting microbiota as a treatment option. Oncotarget, 8(59), 100899-100907.
doi: 10.18632/oncotarget.21284 URL |
| [38] | Ma E. L., Smith A. D., Desai N., Cheung L., Hanscom M., Stoica B. A., Loane D. J., Shea-Donohue T., & Faden A. I. (2017). Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain, Behavior, and Immunity, 66(6), 56-69. |
| [39] |
Ma X., Asif H., Dai L., He Y., Zheng W., Wang D., … Chen X. (2020). Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. Journal of Psychiatric Research, 123(2), 136-144.
doi: 10.1016/j.jpsychires.2020.02.005 URL |
| [40] | MacKenzie N. E., Kowalchuk C., Agarwal S. M., Costa-Dookhan K. A., Caravaggio F., Gerretsen P., Chintoh A., Remington G. J., Taylor V. H., Müeller D. J., Graff-Guerrero D., & Hahn M. K. (2018). Antipsychotics, metabolic adverse effects, and cognitive function in schizophrenia. Frontiers in Psychiatry, 9, Article 622. https://doi.org/10.3389/fpsyt.2018.00622 |
| [41] |
Man L., Lv X., Du X.-D., Yin G., Zhu X., Zhang Y., Soares J. C., Yang X.-N., Chen X., & Zhang X. Y. (2018). Cognitive impairments and low BDNF serum levels in first-episode drug-naive patients with schizophrenia. Psychiatry Research, 263(2), 1-6.
doi: 10.1016/j.psychres.2018.02.034 URL |
| [42] | Manchia M., Fontana A., Panebianco C., Paribello P., Arzedi C., Cossu E., Garzilli M., Montis M. A., Mura A., Pisanu C., Congiu D., Copetti M., Pinna F., Pazienza V., Squassina A., & Carpiniello B. (2021). Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines, 9(8), Article 875 https://doi.org/10.3390/biomedicines9080875 |
| [43] |
Meltzer H. Y., & McGurk S. R. (1999). The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin, 25(2), 233-256.
pmid: 10416729 |
| [44] | Morgan X. C., Tickle T. L., Sokol H., Gevers D., Devaney K. L., Ward D. V., … Huttenhower C. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology, 13(9), 1-18. |
| [45] |
Mörkl S., Butler M. I., Holl A., Cryan J. F., & Dinan T. G. (2020). Probiotics and the microbiota-gut-brain axis: Focus on psychiatry. Current Nutrition Reports, 9(3), 171-182.
doi: 10.1007/s13668-020-00313-5 URL |
| [46] | Munawar N., Ahsan K., Muhammad K., Ahmad A., Anwar M. A., Shah I., Al Ameri A. K., & Al Mughairbi F. (2021). Hidden role of gut microbiome dysbiosis in schizophrenia: Antipsychotics or psychobiotics as therapeutics? International Journal of Molecular Sciences, 22(14), 7671. https://doi.org/10.3390/ijms22147671 |
| [47] |
Nemani K., Ghomi R. H., McCormick B., & Fan X. (2015). Schizophrenia and the gut-brain axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 56(8), 155-160.
doi: 10.1016/j.pnpbp.2014.08.018 URL |
| [48] |
Ng Q. X., Soh A. Y. S., Venkatanarayanan N., Ho C. Y. X., Lim D. Y., & Yeo W.-S. (2019). A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology, 78(1), 1-6.
doi: 10.1159/000498862 URL |
| [49] |
Nguyen T. T., Kosciolek T., Maldonado Y., Daly R. E., Martin A. S., McDonald D., Knight R., & Jeste D. V. (2019). Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophrenia Research, 204(9), 23-29.
doi: 10.1016/j.schres.2018.09.014 URL |
| [50] | Oliphant K., Ali M., D’Souza M., Hughes P. D., Sulakhe D., Wang A. Z., Xie B., Yeasin R., Msall M. E., Andrews B., & Claud E. C. (2021). Bacteroidota and Lachnospiraceae integration into the gut microbiome at key time points in early life are linked to infant neurodevelopment. Gut Microbes, 13(1), Article 1997560. https://doi.org/10.1080/19490976.2021.1997560 |
| [51] | Ong I. M., Gonzalez J. G., McIlwain S. J., Sawin E. A., Schoen A. J., Adluru N., Alexander A. L., & Yu J.-P. J. (2018). Gut microbiome populations are associated with structure-specific changes in white matter architecture. Translational Psychiatry, 8(1), 6. |
| [52] | Pedraz-Petrozzi B., Elyamany O., Rummel C., & Mulert C. (2020). Effects of inflammation on the kynurenine pathway in schizophrenia—A systematic review. Journal of Neuroinflammation, 17(1), 56. |
| [53] |
Rodrigues-Amorim D., Rivera-Baltanás T., Regueiro B., Spuch C., de Las Heras M. E., Vázquez-Noguerol Méndez R., Nieto-Araujo M., Barreiro-Villar C., Olivares J. M., & Agís-Balboa R. C. (2018). The role of the gut microbiota in schizophrenia: Current and future perspectives. The World Journal of Biological Psychiatry, 19(8), 571-585.
doi: 10.1080/15622975.2018.1433878 pmid: 29383983 |
| [54] |
Rogers G., Keating D. J., Young R. L., Wong M.-L., Licinio J., & Wesselingh S. (2016). From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Molecular Psychiatry, 21(6), 738-748.
doi: 10.1038/mp.2016.50 pmid: 27090305 |
| [55] |
Schwarcz R., Bruno J. P., Muchowski P. J., & Wu H. Q. (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nature Review Neuroscience, 13(7), 465-477.
doi: 10.1038/nrn3257 URL |
| [56] |
Schwarz E., Maukonen J., Hyytiäinen T., Kieseppä T., Orešič M., Sabunciyan S., Mantere O., Saarela M., Yolken R., & Suvisaari J. (2018). Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophrenia Research, 192(4), 398-403.
doi: 10.1016/j.schres.2017.04.017 URL |
| [57] |
Seeman M. V. (2021). History of the dopamine hypothesis of antipsychotic action. World Journal of Psychiatry, 11(7), 355-364.
doi: 10.5498/wjp.v11.i7.355 pmid: 34327128 |
| [58] |
Sharon G., Sampson T. R., Geschwind D. H., & Mazmanian S. K. (2016). The central nervous system and the gut microbiome. Cell, 167(4), 915-932.
doi: S0092-8674(16)31447-7 pmid: 27814521 |
| [59] |
Shen Y., Xu J., Li Z., Huang Y., Yuan Y., Wang J., Zhang M., Hu S., & Liang Y. (2018). Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophrenia Research, 197(1), 470-477.
doi: 10.1016/j.schres.2018.01.002 URL |
| [60] | Singh R. K., Chang H.-W., Yan D., Lee K. M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T. H., Bhutani T., & Liao W. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15(1), 73. |
| [61] |
Stahl S. M. (2018). Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectrums, 23(3), 187-191.
doi: 10.1017/S1092852918001013 URL |
| [62] | Stępnicki P., Kondej M., & Kaczor A. A. (2018). Current concepts and treatments of schizophrenia. Molecules, 23(8), Article 2087. https://doi.org/10.3390/molecules23082087 |
| [63] |
Strandwitz P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Research, 1693(3), 128-133.
doi: 10.1016/j.brainres.2018.03.015 URL |
| [64] | Szeligowski T., Yun A. L., Lennox B. R., & Burnet P. W. (2020). The gut microbiome and schizophrenia: The current state of the field and clinical applications. Frontiers in Psychiatry, 11, Article 156. https://doi.org/10.3389/fpsyt.2020.00156 |
| [65] |
Tillisch K., Mayer E., Gupta A., Gill Z., Brazeilles R., Le Nevé B., van Hylckama Vlieg J. E., Guyonnet D., Derrien M., & Labus J. (2017). Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosomatic Medicine, 79(8), 905-913.
doi: 10.1097/PSY.0000000000000493 pmid: 28661940 |
| [66] | Upthegrove R., & Khandaker G. M. (2019). Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Neuroinflammation and schizophrenia. (Vol.44, pp. 49-66). New York: Springer International Publishing. |
| [67] | Vogt N. M., Kerby R. L., Dill-McFarland K. A., Harding S. J., Merluzzi A. P., Johnson S. C., … Rey F. E. (2017). Gut microbiome alterations in Alzheimer’s disease. Scientific Reports, 7(1), 13537. |
| [68] |
Wang Y., Yuan X., Kang Y., & Song X. (2019). Tryptophan-kynurenine pathway as a novel link between gut microbiota and schizophrenia: A review. Tropical Journal of Pharmaceutical Research, 18(4), 897-905.
doi: 10.4314/tjpr.v18i4.30 URL |
| [69] | Williams B. B., van Benschoten A. H., Cimermancic P., Donia M. S., Zimmermann M., Taketani M., Ishihara A., Kashyap P. C., Fraser J. S., & Fischbach M. A. (2014). Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host & Microbe, 16(4), 495-503. |
| [70] | Xu R., Wu B., Liang J., He F., Gu W., Li K., … Wang M. (2020). Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain, Behavior, and Immunity, 85(6), 120-127. |
| [71] | Yuan X., Wang Y., Li X., Jiang J., Kang Y., Pang L., Zhang P., Li A., Lv L., Andreassen O. A., Fan X., Hu S., & Song X. (2021). Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: A 24-week follow-up study. Transl Psychiatry, 11(1), 422. |
| [72] | Zeng C., Yang P., Cao T., Gu Y., Li N., Zhang B., Xu P., Liu Y., Luo Z., & Cai H. (2021). Gut microbiota: An intermediary between metabolic syndrome and cognitive deficits in schizophrenia. Progress in Neuro- Psychopharmacology and Biological Psychiatry, 106, Article 110097. https://doi.org/10.1016/j.pnpbp.2020.110097 |
| [73] |
Zhang J., Guo Z., Xue Z., Sun Z., Zhang M., Wang L., … Zhang H. (2015). A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. The ISME Journal, 9(9), 1979-1990.
doi: 10.1038/ismej.2015.11 URL |
| [74] | Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y., … Xie P. (2019). The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Science Advances, 5(2), Article eaau8317. https://doi.org/10.1126/sciadv.aau8317 |
| [75] |
Zhu F., Guo R., Wang W., Ju Y., Wang Q., Ma Q., … Ma X. (2020). Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Molecular Psychiatry, 25(11), 2905-2918.
doi: 10.1038/s41380-019-0475-4 URL |
| [76] | Zhu F., Ju Y., Wang W., Wang Q., Guo R., Ma Q., … Ma X. (2020). Metagenome-wide association of gut microbiome features for schizophrenia. Nature Communications, 11(1), 1612. |
| [77] |
Zou Y., Xue W., Luo G., Deng Z., Qin P., Guo R., … Xiao L. (2019). 1, 520 reference genomes from cultivated human gut bacteria enable functional microbiome analyses. Nature Biotechnology, 37(2), 179-185.
doi: 10.1038/s41587-018-0008-8 URL |
| [1] | 夏行之, 夏海森, 杜向东. 精神分裂症患者对情绪图片唤醒研究[J]. 心理科学进展, 2023, 31(suppl.): 131-131. |
| [2] | 彭玉佳, 王愉茜, 路迪. 基于生物运动的社交焦虑者情绪加工与社会意图理解负向偏差机制[J]. 心理科学进展, 2023, 31(6): 905-914. |
| [3] | 梁飞, 江瑶, 肖婷炜, 董洁, 王福顺. 基本情绪的神经基础:来自fMRI与机器视觉技术研究的证据[J]. 心理科学进展, 2022, 30(8): 1832-1843. |
| [4] | 李谷静, 张丽蓉, 米莉, 贺辉, 卢竞, 罗程, 尧德中. 舞动治疗:一种自下而上的精神分裂症干预探索[J]. 心理科学进展, 2021, 29(8): 1371-1380. |
| [5] | 欧华星, 陈伟海. 多巴胺D2受体参与调节感觉门控的机制[J]. 心理科学进展, 2021, 29(6): 1030-1041. |
| [6] | 郑泓, 蒲城城, 王毅, 陈楚侨. 基于脑结构像的精神分裂症机器学习分类[J]. 心理科学进展, 2020, 28(2): 252-265. |
| [7] | 王盛, 陈雅弘, 王锦琰. 动物前注意加工模型的建立及评价: 基于精神类疾病损伤[J]. 心理科学进展, 2020, 28(12): 2027-2039. |
| [8] | 曹艺, 杨小虎. 精神分裂症患者的语音感知[J]. 心理科学进展, 2019, 27(6): 1025-1035. |
| [9] | 邓潇斐, 郭建友. Parvalbumin阳性中间神经元缺陷在精神分裂症病理机制中的作用[J]. 心理科学进展, 2018, 26(11): 1992-2002. |
| [10] | 朱传林;李萍;罗文波;齐正阳;何蔚祺. 精神分裂症患者的情绪调节[J]. 心理科学进展, 2016, 24(4): 556-572. |
| [11] | 郭亚飞;金盛华;王建平;吴林桦;艾迪玛. DSM-5精神分裂症谱系的新变化:类别与维度之争[J]. 心理科学进展, 2015, 23(8): 1428-1436. |
| [12] | 薛晓芳;李曼;王玮文;邵枫. 母婴分离的动物模型及其神经生物学机制[J]. 心理科学进展, 2013, 21(6): 990-998. |
| [13] | 吴超;吴玺宏;李量. 精神分裂症患者在听觉掩蔽环境下的言语识别[J]. 心理科学进展, 2013, 21(6): 958-964. |
| [14] | 杜忆;李量. 对听感觉运动门控自上而下调节的动物模型和神经机制[J]. 心理科学进展, 2011, 19(7): 944-958. |
| [15] | 史艳芳;陈楚侨. 精神分裂症谱系中的快感缺乏[J]. 心理科学进展, 2010, 18(9): 1430-1439. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||