

CN 11-1911/B


Acta Psychologica Sinica ›› 2022, Vol. 54 ›› Issue (6): 604-612.doi: 10.3724/SP.J.1041.2022.00604
• Reports of Empirical Studies • Previous Articles Next Articles
ZHOU Ping1, XIAO Hua1, LI Yonghui2,3, DONG Xinwen2( )
)
Received:2021-08-17
Online:2022-04-26
Contact:
DONG Xinwen
E-mail:dongxw@psych.ac.cn
Supported by:ZHOU Ping, XIAO Hua, LI Yonghui, DONG Xinwen. (2022). Sustained hyperarousal induced by acute stress in tryptophan-hydroxylase-2 genetic deficient male mice. Acta Psychologica Sinica, 54(6), 604-612.
Add to citation manager EndNote|Ris|BibTeX
URL: https://journal.psych.ac.cn/acps/EN/10.3724/SP.J.1041.2022.00604
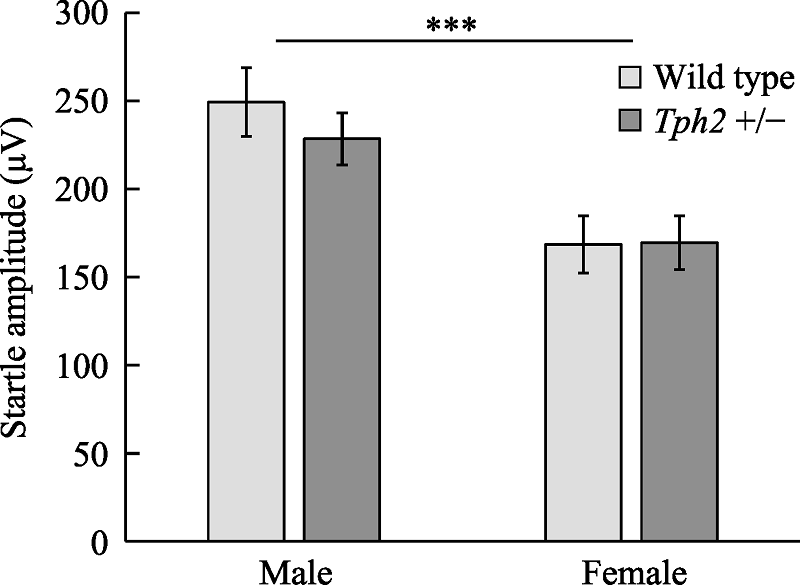
Figure 1. Effects of sex and genotype on the amplitude of auditory startle reflex in mice. Note. The error bar is standard error of mean (SEM). Cases in each group: male wild, n = 26; Male Tph2 +/-, n = 37; Female wild type, n = 31; Female Tph2 +/-, n = 29. The baseline value of startle reflex in male wild-type mice was significantly higher than that in female mice, *** p < 0.001, and there was no difference in baseline value of startle reflex between mice of the same sex and different genotypes.
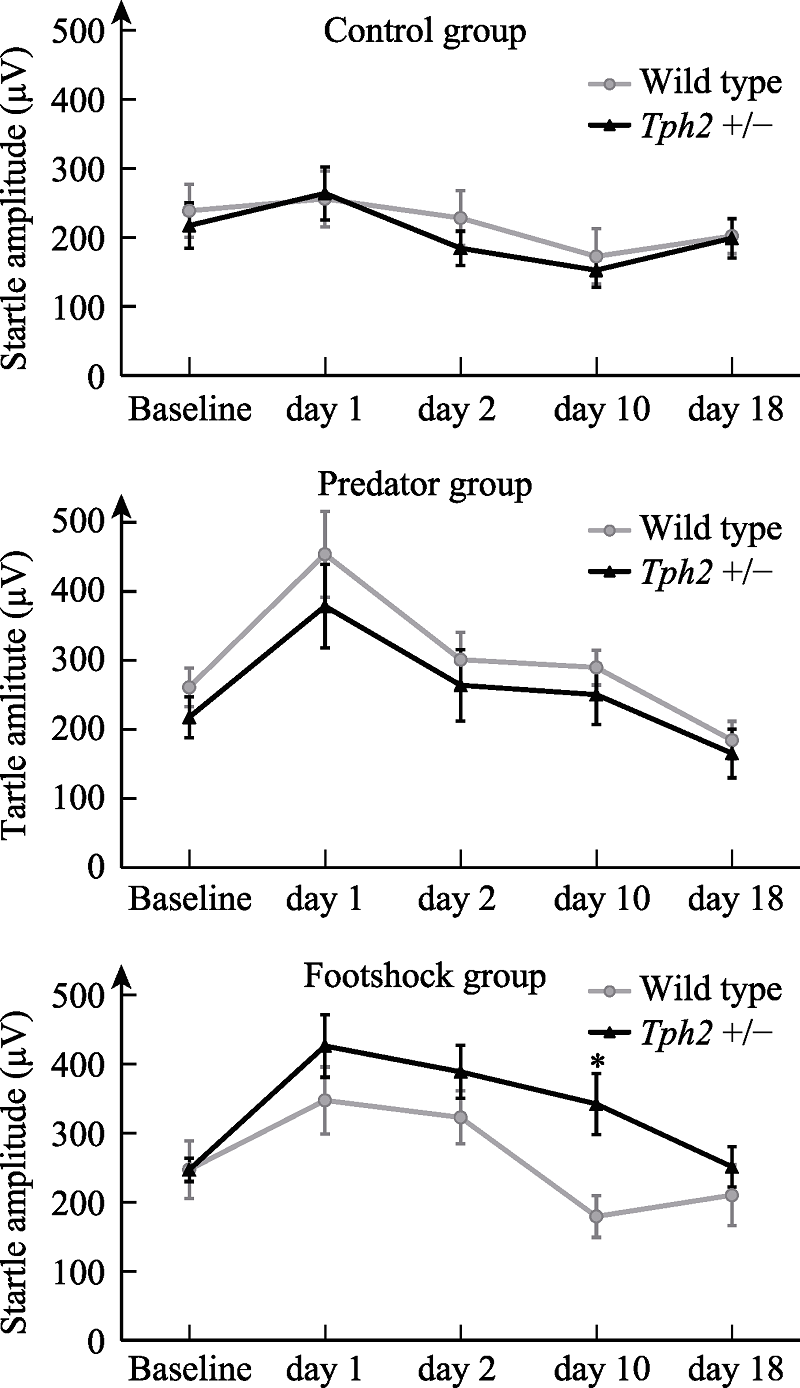
Figure 2. Changes of auditory startle reflex amplitude in male mice before and after stress. Note. The error bar is standard error of mean (SEM). Cases in each group: wild type control group, n=8, predator group, n=9, shock group, n=9; Tph2 +/- control group, n = 13, predator group, n = 10, shock group, n = 14. The * mark in the figure indicates the difference of startle reflex amplitude among male mice of different genotypes 10 days after electric shock stress, * p < 0.05.
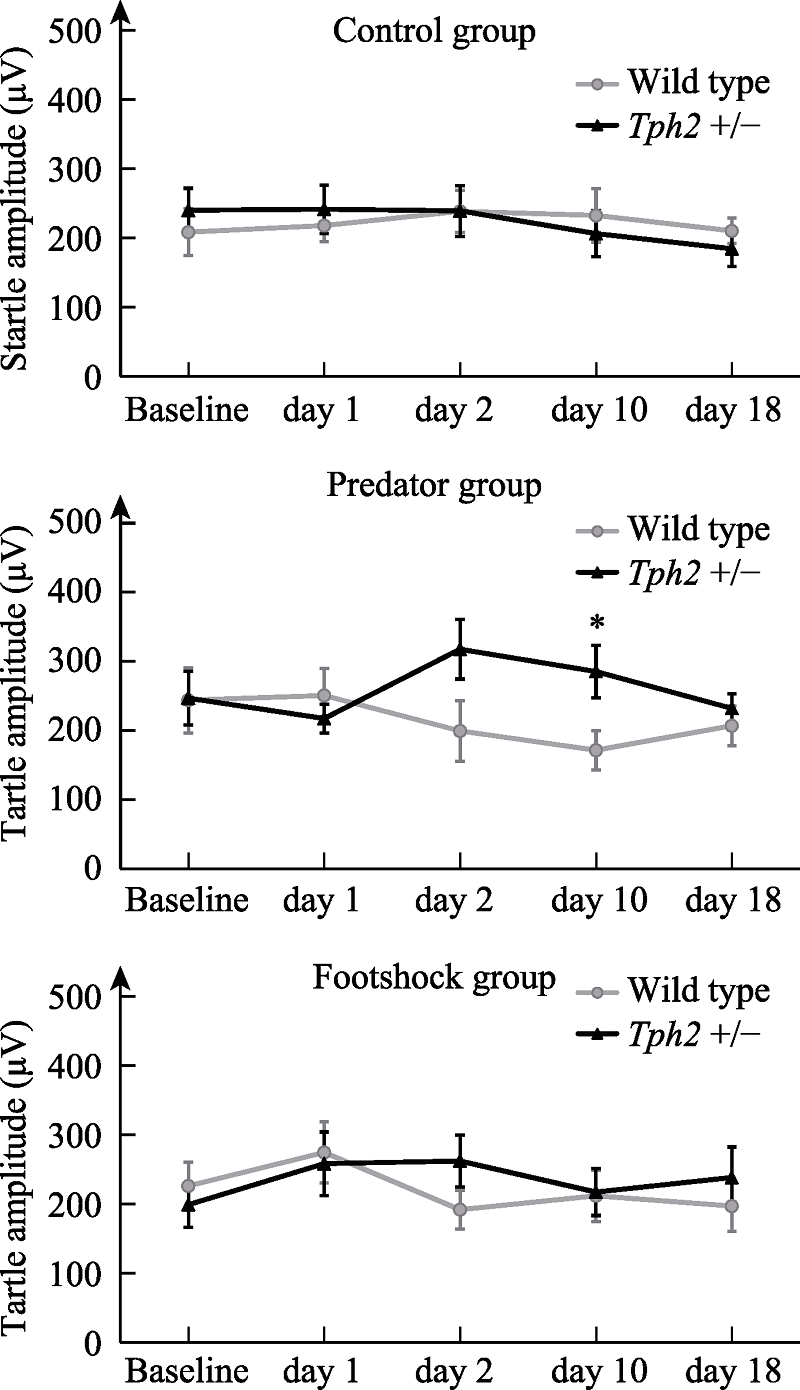
Figure 3. Change of amplitude of auditory startle reflex in female mice before and after stress. Note. The error bar is standard error of mean (SEM). Cases in each group: wild type control group, n = 12, predator group, n = 8, shock group, n = 11; Tph2 +/- Female control group, n = 12, predator group, n = 6, shock group, n = 11. The * mark in the figure indicates the difference of startle reflex amplitude among female mice of different genotypes 10 days after predator exposure stress, * p < 0.05.
| [1] |
Abela, A. R., Browne, C. J., Sargin, D., Prevot, T. D., Ji, X. D., Li, Z., Lambe, E. K., & Fletcher, P. J. (2020). Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology, 168, 107985. https://doi.org/10.1016/j.neuropharm.2020.107985
doi: 10.1016/j.neuropharm.2020.107985 URL |
| [2] |
Agarwal, T. M., Muneer, M., Asim, M., Awad, M., Afzal, Y., Al-Thani, H., Alhassan, A., Mollazehi, M., & El-Menyar, A. (2020). Psychological trauma in different mechanisms of traumatic injury: A hospital-based cross-sectional study. PLoS ONE, 15(11), e0242849. https://doi.org/10.1371/journal.pone.0242849
doi: 10.1371/journal.pone.0242849 URL |
| [3] | Akiki, T. J., & Abdallah, C. G. (2018). Are There Effective Psychopharmacologic Treatments for PTSD? The Journal of Clinical Psychiatry, 80(3), 18ac12473. https://doi.org/10.4088/JCP.18ac12473 |
| [4] | American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596 |
| [5] |
Auth, C. S., Weidner, M. T., Popp, S., Strekalova, T., Schmitt-Böhrer, A. G., van den Hove, D. L., Lesch, K.-P., & Waider, J. (2018). Differential anxiety-related behaviours and brain activation in Tph2-deficient female mice exposed to adverse early environment. European Neuropsychopharmacology, 28(11), 1270-1283. https://doi.org/10.1016/j.euroneuro.2018.07.103
doi: 10.1016/j.euroneuro.2018.07.103 URL |
| [6] |
Bernabe, C. S., Caliman, I. F., Truitt, W. A., Molosh, A. I., Lowry, C. A., Hay-Schmidt, A., Shekhar, A., & Johnson, P. L. (2020). Using loss- and gain-of-function approaches to target amygdala-projecting serotonergic neurons in the dorsal raphe nucleus that enhance anxiety- related and conditioned fear behaviors. Journal of Psychopharmacology, 34(4), 400-411. https://doi.org/10.1177/0269881119900981
doi: 10.1177/0269881119900981 URL |
| [7] |
Boal, A. L., Vaughan, C. A., Sims, C. S., & Miles, J. N. V. (2017). Measurement invariance across administration mode: Examining the Posttraumatic Stress Disorder (PTSD) Checklist. Psychological Assessment, 29(1), 76-86. https://doi.org/10.1037/pas0000301
doi: 10.1037/pas0000301 URL |
| [8] |
Brivio, P., Sbrini, G., Peeva, P., Todiras, M., Bader, M., Alenina, N., & Calabrese, F. (2018). TPH2 deficiency influences neuroplastic mechanisms and alters the response to an acute stress in a sex specific manner. Frontiers in Molecular Neuroscience, 11, 389. https://doi.org/10.3389/fnmol.2018.00389
doi: 10.3389/fnmol.2018.00389 URL pmid: 30425618 |
| [9] |
Bryant, R. A., Creamer, M., O’Donnell, M., Forbes, D., McFarlane, A. C., Silove, D., & Hadzi-Pavlovic, D. (2017). Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: A network analysis. JAMA Psychiatry, 74(2), 135-142. https://doi.org/10.1001/jamapsychiatry.2016.3470
doi: 10.1001/jamapsychiatry.2016.3470 URL |
| [10] |
Bryant, R. A., Creamer, M., O’Donnell, M., Silove, D., & McFarlane, A. C. (2008). A multisite study of initial respiration rate and heart rate as predictors of posttraumatic stress disorder. The Journal of Clinical Psychiatry, 69(11), 1694-1701. https://doi.org/10.4088/JCP.v69n1104
doi: 10.4088/JCP.v69n1104 URL |
| [11] |
Cao, C., Wang, L., Wang, R., Qing, Y., & Zhang, J. (2014). TPH2 genotype is associated with PTSD’s avoidance symptoms in Chinese female earthquake survivors. Psychiatric Genetics, 24(6), 257-261. https://doi.org/10.1097/YPG.0000000000000048
doi: 10.1097/YPG.0000000000000048 URL |
| [12] |
Cohen, H., Kaplan, Z., Koresh, O., Matar, M. A., Geva, A. B., & Zohar, J. (2011). Early post-stressor intervention with propranolol is ineffective in preventing posttraumatic stress responses in an animal model for PTSD. European Neuropsychopharmacology, 21(3), 230-240. https://doi.org/10.1016/j.euroneuro.2010.11.011
doi: 10.1016/j.euroneuro.2010.11.011 URL |
| [13] | Cohen, H., & Zohar, J. (2004). An animal model of posttraumatic stress disorder:The use of cut-off behavioral criteria. Annals of the New York Academy of Sciences, 1032(1), 167-178. https://doi.org/10.1196/annals.1314.014 |
| [14] |
Coronas, R., Gallardo, O., Moreno, M. J., Suárez, D., García-Parés, G., & Menchón, J. M. (2011). Heart rate measured in the acute aftermath of trauma can predict post-traumatic stress disorder: A prospective study in motor vehicle accident survivors. European Psychiatry, 26(8), 508-512. https://doi.org/10.1016/j.eurpsy.2010.06.006
doi: 10.1016/j.eurpsy.2010.06.006 URL pmid: 20813504 |
| [15] |
da Silva Soares, R., Falconi-Sobrinho, L. L., Almada, R. C., & Coimbra, N. C. (2019). Dorsal raphe nucleus 5-Hydroxytryptamine 2A receptors are critical for the organisation of panic attack-like defensive behaviour and unconditioned fear-induced antinociception elicited by the chemical stimulation of superior colliculus neurons. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 29(7), 858-870. https://doi.org/10.1016/j.euroneuro.2019.05.007
doi: 10.1016/j.euroneuro.2019.05.007 URL |
| [16] | Dong, X., & Li, Y. (2014). Peritraumatic startle response predicts the vulnerability to develop PTSD-like behaviors in mice: A model for peritraumatic dissociation. Frontiers in Behavioral Neuroscience, 8, 14. https://doi.org/10.3389/fnbeh.2014.00014 |
| [17] |
Gelkopf, M., Lapid Pickman, L., Carlson, E. B., & Greene, T. (2019). The dynamic relations among peritraumatic posttraumatic stress symptoms: An experience sampling study during wartime. Journal of Traumatic Stress, 32(1), 119-129. https://doi.org/10.1002/jts.22374
doi: 10.1002/jts.22374 URL pmid: 30720893 |
| [18] |
Gonzales, M., Garrett, C., Chapman, C. D., & Dess, N. K. (2008). Stress-induced attenuation of acoustic startle in low-saccharin- consuming mice. Biological Psychology, 79(2), 193-199. https://doi.org/10.1016/j.biopsycho.2008.04.011
doi: 10.1016/j.biopsycho.2008.04.011 URL pmid: 18538914 |
| [19] |
Greene, T., Gelkopf, M., Fried, E. I., Robinaugh, D. J., & Lapid Pickman, L. (2020). Dynamic network analysis of negative emotions and DSM-5 posttraumatic stress disorder symptom clusters during conflict. Journal of Traumatic Stress, 33(1), 72-83. https://doi.org/10.1002/jts.22433
doi: 10.1002/jts.22433 URL pmid: 31433530 |
| [20] |
Gross, C. T., & Canteras, N. S. (2012). The many paths to fear. Nature Reviews Neuroscience, 13(9), 651-658. https://doi.org/10.1038/nrn3301
doi: 10.1038/nrn3301 URL |
| [21] |
Gutknecht, L., Araragi, N., Merker, S., Waider, J., Sommerlandt, F. M. J., Mlinar, B., Baccini, G., Mayer, U., Proft, F., Hamon, M., Schmitt, A. G., Corradetti, R., Lanfumey, L., & Lesch, K.-P. (2012). Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS ONE, 7(8), e43157. https://doi.org/10.1371/journal.pone.0043157
doi: 10.1371/journal.pone.0043157 URL |
| [22] |
Hubbard, C. S., Ornitz, E., Gaspar, J. X., Smith, S., Amin, J., Labus, J. S., Kilpatrick, L. A., Rhudy, J. L., Mayer, E. A., & Naliboff, B. D. (2011). Modulation of nociceptive and acoustic startle responses to an unpredictable threat in men and women. Pain, 152(7), 1632-1640. https://doi.org/10.1016/j.pain.2011.03.001
doi: 10.1016/j.pain.2011.03.001 URL |
| [23] |
Jacobsen, J. P. R., Siesser, W. B., Sachs, B. D., Peterson, S., Cools, M. J., Setola, V., Folgering, J. H. A., Flik, G., & Caron, M. G. (2012). Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of- function mice. Molecular Psychiatry, 17(7), 694-704. https://doi.org/10.1038/mp.2011.50
doi: 10.1038/mp.2011.50 URL pmid: 21537332 |
| [24] |
Kaehler, S. T., Singewald, N., Sinner, C., Thurnher, C., & Philippu, A. (2000). Conditioned fear and inescapable shock modify the release of serotonin in the locus coeruleus. Brain Research, 859(2), 249-254. https://doi.org/10.1016/s0006-8993(00)01967-3
URL pmid: 10719071 |
| [25] |
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., & Nelson, C. B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
doi: 10.1001/archpsyc.1995.03950240066012 URL pmid: 7492257 |
| [26] |
Kilpatrick, D. G., Resnick, H. S., Milanak, M. E., Miller, M. W., Keyes, K. M., & Friedman, M. J. (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26(5), 537-547. https://doi.org/10.1002/jts.21848
doi: 10.1002/jts.21848 URL pmid: 24151000 |
| [27] |
Kobayashi, I., & Mellman, T. A. (2012). Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behavioral Sleep Medicine, 10(3), 180-190. https://doi.org/10.1080/15402002.2011.654296
doi: 10.1080/15402002.2011.654296 URL pmid: 22742436 |
| [28] |
Kolter, J. F., Hildenbrand, M. F., Popp, S., Nauroth, S., Bankmann, J., Rother, L., Waider, J., Deckert, J., Asan, E., Jakob, P. M., Lesch, K.-P., & Schmitt-Böhrer, A. (2021). Serotonin transporter genotype modulates resting state and predator stress-induced amygdala perfusion in mice in a sex-dependent manner. PloS One, 16(2), e0247311. https://doi.org/10.1371/journal.pone.0247311
doi: 10.1371/journal.pone.0247311 URL |
| [29] |
Koresh, O., Kaplan, Z., Zohar, J., Matar, M. A., Geva, A. B., & Cohen, H. (2016). Distinctive cardiac autonomic dysfunction following stress exposure in both sexes in an animal model of PTSD. Behavioural Brain Research, 308, 128-142. https://doi.org/10.1016/j.bbr.2016.04.024
doi: 10.1016/j.bbr.2016.04.024 URL pmid: 27105958 |
| [30] |
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1990). Emotion, attention, and the startle reflex. Psychological Review, 97(3), 377-395.
pmid: 2200076 |
| [31] |
Lieb, M. W., Weidner, M., Arnold, M. R., Loupy, K. M., Nguyen, K. T., Hassell, J. E., Schnabel, K. S., Kern, R., Day, H. E. W., Lesch, K.-P., Waider, J., & Lowry, C. A. (2019). Effects of maternal separation on serotonergic systems in the dorsal and median raphe nuclei of adult male Tph2-deficient mice. Behavioural Brain Research, 373, 112086. https://doi.org/10.1016/j.bbr.2019.112086
doi: 10.1016/j.bbr.2019.112086 URL |
| [32] |
Linthorst, A. C. E., & Reul, J. M. (2008). Stress and the brain: Solving the puzzle using microdialysis. Pharmacology Biochemistry and Behavior, 90(2), 163-173. https://doi.org/10.1016/j.pbb.2007.09.019
URL pmid: 18028991 |
| [33] |
Liu, Y., Jiang, Y., Si, Y., Kim, J.-Y., Chen, Z.-F., & Rao, Y. (2011). Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature, 472(7341), 95-99. https://doi.org/10.1038/nature09822
doi: 10.1038/nature09822 URL |
| [34] |
McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. https://doi.org/10.1177/2470547017692328
doi: 10.1177/2470547017692328 URL |
| [35] |
McQuade, R., & Sharp, T. (2002). Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. Journal of Neurochemistry, 69(2), 791-796. https://doi.org/10.1046/j.1471-4159.1997.69020791.
doi: 10.1046/j.1471-4159.1997.69020791.x URL |
| [36] |
Monti, J. M. (2011). Serotonin control of sleep-wake behavior. Sleep Medicine Reviews, 15(4), 269-281. https://doi.org/10.1016/j.smrv.2010.11.003
doi: 10.1016/j.smrv.2010.11.003 URL |
| [37] |
Mosienko, V., Matthes, S., Hirth, N., Beis, D., Flinders, M., Bader, M., Hansson, A. C., & Alenina, N. (2014). Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology, 85, 73-80. https://doi.org/10.1016/j.neuropharm.2014.05.015
doi: 10.1016/j.neuropharm.2014.05.015 URL |
| [38] |
Russo, A. M., Lawther, A. J., Prior, B. M., Isbel, L., Somers, W. G., Lesku, J. A., Richdale, A. L., Dissanayake, C., Kent, S., Lowry, C. A., & Hale, M. W. (2019). Social approach, anxiety, and altered tryptophan hydroxylase 2 activity in juvenile BALB/c and C57BL/6J mice. Behavioural Brain Research, 359, 918-926. https://doi.org/10.1016/j.bbr.2018.06.019
doi: 10.1016/j.bbr.2018.06.019 URL |
| [39] |
Sachs, B. D., Ni, J. R., & Caron, M. G. (2015). Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proceedings of the National Academy of Sciences, 112(8), 2557-2562. https://doi.org/10.1073/pnas.1416866112
doi: 10.1073/pnas.1416866112 URL |
| [40] |
Savelieva, K. V., Zhao, S., Pogorelov, V. M., Rajan, I., Yang, Q., Cullinan, E., & Lanthorn, T. H. (2008). Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE, 3(10), e3301. https://doi.org/10.1371/journal.pone.0003301
doi: 10.1371/journal.pone.0003301 URL |
| [41] |
Sengupta, A., & Holmes, A. (2019). A discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron, 103(3), 489-505.e7. https://doi.org/10.1016/j.neuron.2019.05.029
doi: S0896-6273(19)30482-9 URL pmid: 31204082 |
| [42] |
Shaikh al arab, A., Guédon-Moreau, L., Ducrocq, F., Molenda, S., Duhem, S., Salleron, J., Chaudieu, I., Bert, D., Libersa, C., & Vaiva, G. (2012). Temporal analysis of heart rate variability as a predictor of post traumatic stress disorder in road traffic accidents survivors. Journal of Psychiatric Research, 46(6), 790-796. https://doi.org/10.1016/j.jpsychires.2012.02.006
doi: 10.1016/j.jpsychires.2012.02.006 URL pmid: 22425487 |
| [43] |
Sherin, J. E., & Nemeroff, C. B. (2011). Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues in Clinical Neuroscience, 13(3), 263-278. https://doi.org/10.31887/DCNS.2011.13.2/jsherin
doi: 10.31887/DCNS.2011.13.2/jsherin URL |
| [44] | Sijbrandij, M., Kleiboer, A., Bisson, J. I., Barbui, C., & Cuijpers, P. (2015). Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: A systematic review and meta-analysis. The Lancet. Psychiatry, 2(5), 413-421. https://doi.org/10.1016/S2215-0366(14)00121-7 |
| [45] |
Smith, K. L., Kassem, M. S., Clarke, D. J., Kuligowski, M. P., Bedoya-Pérez, M. A., Todd, S. M., Lagopoulos, J., Bennett, M. R., & Arnold, J. C. (2019). Microglial cell hyper-ramification and neuronal dendritic spine loss in the hippocampus and medial prefrontal cortex in a mouse model of PTSD. Brain, Behavior, and Immunity, 80, 889-899. https://doi.org/10.1016/j.bbi.2019.05.042
doi: 10.1016/j.bbi.2019.05.042 URL |
| [46] |
Stam, R. (2007). PTSD and stress sensitisation: A tale of brain and body Part 2: Animal models. Neuroscience & Biobehavioral Reviews, 31(4), 558-584. https://doi.org/10.1016/j.neubiorev.2007.01.001
doi: 10.1016/j.neubiorev.2007.01.001 URL |
| [47] |
Strekalova, T., Svirin, E., Waider, J., Gorlova, A., Cespuglio, R., Kalueff, A., Pomytkin, I., Schmitt-Boehrer, A. G., Lesch, K.-P., & Anthony, D. C. (2021). Altered behaviour, dopamine and norepinephrine regulation in stressed mice heterozygous in TPH2 gene. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 108, 110155. https://doi.org/10.1016/j.pnpbp.2020.110155
doi: 10.1016/j.pnpbp.2020.110155 URL pmid: 33127424 |
| [48] |
Tao, S., Chattun, M. R., Yan, R., Geng, J., Zhu, R., Shao, J., Lu, Q., & Yao, Z. (2018). TPH-2 gene polymorphism in major depressive disorder patients with early-wakening symptom. Frontiers in Neuroscience, 12, 827. https://doi.org/10.3389/fnins.2018.00827
doi: 10.3389/fnins.2018.00827 URL |
| [49] | Thakur, A., Choudhary, D., Kumar, B., & Chaudhary, A. (2021). A review on post-traumatic stress disorder (PTSD): “Symptoms, therapies and recent case studies.” Current Molecular Pharmacology. https://doi.org/10.2174/1874467214666210525160944 |
| [50] |
Török, B., Sipos, E., Pivac, N., & Zelena, D. (2019). Modelling posttraumatic stress disorders in animals. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 90, 117-133. https://doi.org/10.1016/j.pnpbp.2018.11.013
doi: 10.1016/j.pnpbp.2018.11.013 URL |
| [51] |
Verona, E., & Kilmer, A. (2007). Stress exposure and affective modulation of aggressive behavior in men and women. Journal of Abnormal Psychology, 116(2), 410-421. https://doi.org/10.1037/0021-843X.116.2.410
doi: 10.1037/0021-843X.116.2.410 URL |
| [52] | Voulo, M. E., & Parsons, R. G. (2017). Response-specific sex difference in the retention of fear extinction. Learning & Memory, 24(6), 245-251. https://doi.org/10.1101/lm.045641.117 |
| [53] |
Voulo, M. E., & Parsons, R. G. (2019). Gonadal hormone fluctuations do not affect the expression or extinction of fear-potentiated startle in female mice. Behavioral Neuroscience, 133(5), 517-526. https://doi.org/10.1037/bne0000324
doi: 10.1037/bne0000324 URL |
| [54] |
Walther, D. J., Peter, J.-U., Bashammakh, S., Hörtnagl, H., Voits, M., Fink, H., & Bader, M. (2003). Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science, 299(5603), 76. https://doi.org/10.1126/science.1078197
URL pmid: 12511643 |
| [55] |
Wankhar, W., Syiem, D., Pakyntein, C. L., Thabah, D., & Sunn, S. E. (2020). Effect of 5-HT2C receptor agonist and antagonist on chronic unpredictable stress (CUS) - Mediated anxiety and depression in adolescent Wistar albino rat: Implicating serotonin and mitochondrial ETC-I function in serotonergic neurotransmission. Behavioural Brain Research, 393, 112780. https://doi.org/10.1016/j.bbr.2020.112780
doi: 10.1016/j.bbr.2020.112780 URL |
| [56] |
Weidner, M. T., Lardenoije, R., Eijssen, L., Mogavero, F., de Groodt, L. P. M. T., Popp, R., Palme, F., Förstner, K. U., Strekalova, T., Steinbusch, H. W. M., Schmitt-Böhrer, A. G., Glennon, J. C., Waider, J., van den Hove, D. L. A., & Lesch, K.-P. (2019). Identification of cholecystokinin by genome-wide profiling as potential mediator of serotonin-dependent behavioral effects of maternal separation in the amygdala. Frontiers in Neuroscience, 13, 460. https://doi.org/10.3389/fnins.2019.00460
doi: 10.3389/fnins.2019.00460 URL |
| [57] |
Xu, Z., Reynolds, G. P., Yuan, Y., Shi, Y., Pu, M., & Zhang, Z. (2016). TPH-2 polymorphisms interact with early life stress to influence response to treatment with antidepressant drugs. International Journal of Neuropsychopharmacology, 19(11), pyw070. https://doi.org/10.1093/ijnp/pyw070
doi: 10.1093/ijnp/pyw070 URL |
| [58] | Young, S. (2013). Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. Journal of Psychiatry & Neuroscience, 38(5), 294-305. https://doi.org/10.1503/jpn.120209 |
| [59] |
Zoladz, P. R., D’Alessio, P. A., Seeley, S. L., Kasler, C. D., Goodman, C. S., Mucher, K. E., Allison, A. S., Smith, I. F., Dodson, J. L., Stoops, T. S., & Rorabaugh, B. R. (2019). A predator-based psychosocial stress animal model of PTSD in females: Influence of estrous phase and ovarian hormones. Hormones and Behavior, 115, 104564. https://doi.org/10.1016/j.yhbeh.2019.104564
doi: 10.1016/j.yhbeh.2019.104564 URL |
| [1] | LI Jiangna; AN Shucheng; LI Zhen. Orbitofrontal Cortex 5-HT1A Receptor Modulate Glutamate and GABA in Depression Induced by Chronic Unpredictable Mild Stress [J]. Acta Psychologica Sinica, 2015, 47(10): 1269-1278. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||