

CN 11-1911/B
主办:中国心理学会
中国科学院心理研究所
出版:科学出版社


心理学报 ›› 2024, Vol. 56 ›› Issue (10): 1313-1327.doi: 10.3724/SP.J.1041.2024.01313 cstr: 32110.14.2024.01313
• 研究报告 • 下一篇
收稿日期:2024-01-15
发布日期:2024-07-10
出版日期:2024-10-25
通讯作者:
涂毅恒, E-mail: tuyh@psych.ac.cn基金资助:
QIU Yi1,2, CHANG Xiang-Yu1, TU Yi-Heng1,2( )
)
Received:2024-01-15
Online:2024-07-10
Published:2024-10-25
摘要:
当前经颅直流电刺激(transcranial direct current stimulation, tDCS)的镇痛效果不佳且存在较大的个体差异, 可能与疼痛神经网络的复杂性及当前tDCS调控靶点单一有关。为提高tDCS缓解疼痛的效果, 本研究使用双盲、随机对照的实验设计, 采用双靶点tDCS技术, 对背外侧前额叶皮层(dorsolateral prefrontal cortex, DLPFC)和初级运动皮层(primary motor cortex, M1)进行同步刺激, 通过调节DLPFC和M1所属不同的疼痛传导通路, 探究双靶点tDCS的镇痛效果及优势。 结果表明, 与假刺激对照组相比, 双靶点tDCS组对中等强度短时热痛、辣椒素诱发的持续性疼痛以及压痛阈限具有显著的调控效应, 且调控效应优于单靶点tDCS组。同时, 双靶点tDCS的镇痛效应与被试的疼痛恐惧特质显著相关, 即被试疼痛恐惧量表评分越高, 镇痛效果越好。综上, 本研究揭示了双靶点tDCS对短时热痛和持续性疼痛的稳定镇痛效果, 支持了疼痛神经网络的并行处理理论, 也为双靶点tDCS在疼痛治疗中的潜在应用提供了科学依据。
中图分类号:
邱义, 常香玉, 涂毅恒. (2024). 双靶点经颅直流电刺激调控短时和持续性疼痛:一项双盲、随机对照研究. 心理学报, 56(10), 1313-1327.
QIU Yi, CHANG Xiang-Yu, TU Yi-Heng. (2024). Analgesic effect of dual-target transcranial direct current stimulation on transient pain and sustained pain: A double-blind, randomized controlled study. Acta Psychologica Sinica, 56(10), 1313-1327.
| 变量 | lDLPFC+rM1 (n = 21) | LDLPFC (n = 20) | rM1 (n = 20) | 假刺激组 (n = 19) | 统计指标 | |
|---|---|---|---|---|---|---|
| F/χ2 | p-fdr | |||||
| 年龄 | 23.71 ± 0.58 | 24.10 ± 0.60 | 22.30 ± 0.60 | 24.50 ± 0.61 | F(3, 76) = 2.66 | 0.054 |
| 性别(女/男) | 10/11 | 11/9 | 10/10 | 9/10 | χ2(3) = 0.3 | 0.960 |
| MP刺激强度(℃) | 46.00 ± 0.33 | 45.88 ± 0.34 | 45.70 ± 0.34 | 45.92 ± 0.35 | F(3, 76) = 0.14 | 0.935 |
| HP刺激强度(℃) | 47.71 ± 0.28 | 47.30 ± 0.28 | 47.45 ± 0.28 | 47.82 ± 0.29 | F(3, 76) = 0.69 | 0.562 |
| 疼痛恐惧量表 | 91.24 ± 3.31 | 95.40 ± 3.39 | 100.70 ± 3.39 | 95.11 ± 3.48 | F(3, 76) = 1.34 | 0.268 |
| 疼痛灾难化量表 | 24.47 ± 2.09 | 24.60 ± 2.14 | 24.95 ± 2.14 | 25.21 ± 2.20 | F(3, 76) = 0.02 | 0.995 |
| 疼痛敏感性量表 | 3.20 ± 0.28 | 3.81 ± 0.29 | 3.41 ± 0.29 | 3.49 ± 0.29 | F(3, 76) = 0.81 | 0.495 |
| 状态焦虑量表 | 33.95 ± 1.82 | 36.35 ± 1.87 | 32.15 ± 1.87 | 36.00 ± 1.92 | F(3, 76) = 1.09 | 0.360 |
| 负性情绪量表 (后测−前测) | 1.19 ± 1.13 | −0.30 ± 1.15 | −1.15 ± 1.15 | −1.12 ± 1.18 | F(3, 76) = 0.9 | 0.436 |
| 正性情绪量表 (后测−前测) | −4.00 ± 1.09 | −3.45 ± 1.12 | −3.00 ± 1.12 | −2.74 ± 1.15 | F(3, 76) = 0.25 | 0.863 |
| tDCS感觉总分 | 16.86 ± 0.75 | 17.05 ± 0.77 | 17.55 ± 0.77 | 19.11 ± 0.78 | F(3, 76) = 1.71 | 0.171 |
表1 各tDCS组被试的人口学变量和量表统计结果(均值±标准误)
| 变量 | lDLPFC+rM1 (n = 21) | LDLPFC (n = 20) | rM1 (n = 20) | 假刺激组 (n = 19) | 统计指标 | |
|---|---|---|---|---|---|---|
| F/χ2 | p-fdr | |||||
| 年龄 | 23.71 ± 0.58 | 24.10 ± 0.60 | 22.30 ± 0.60 | 24.50 ± 0.61 | F(3, 76) = 2.66 | 0.054 |
| 性别(女/男) | 10/11 | 11/9 | 10/10 | 9/10 | χ2(3) = 0.3 | 0.960 |
| MP刺激强度(℃) | 46.00 ± 0.33 | 45.88 ± 0.34 | 45.70 ± 0.34 | 45.92 ± 0.35 | F(3, 76) = 0.14 | 0.935 |
| HP刺激强度(℃) | 47.71 ± 0.28 | 47.30 ± 0.28 | 47.45 ± 0.28 | 47.82 ± 0.29 | F(3, 76) = 0.69 | 0.562 |
| 疼痛恐惧量表 | 91.24 ± 3.31 | 95.40 ± 3.39 | 100.70 ± 3.39 | 95.11 ± 3.48 | F(3, 76) = 1.34 | 0.268 |
| 疼痛灾难化量表 | 24.47 ± 2.09 | 24.60 ± 2.14 | 24.95 ± 2.14 | 25.21 ± 2.20 | F(3, 76) = 0.02 | 0.995 |
| 疼痛敏感性量表 | 3.20 ± 0.28 | 3.81 ± 0.29 | 3.41 ± 0.29 | 3.49 ± 0.29 | F(3, 76) = 0.81 | 0.495 |
| 状态焦虑量表 | 33.95 ± 1.82 | 36.35 ± 1.87 | 32.15 ± 1.87 | 36.00 ± 1.92 | F(3, 76) = 1.09 | 0.360 |
| 负性情绪量表 (后测−前测) | 1.19 ± 1.13 | −0.30 ± 1.15 | −1.15 ± 1.15 | −1.12 ± 1.18 | F(3, 76) = 0.9 | 0.436 |
| 正性情绪量表 (后测−前测) | −4.00 ± 1.09 | −3.45 ± 1.12 | −3.00 ± 1.12 | −2.74 ± 1.15 | F(3, 76) = 0.25 | 0.863 |
| tDCS感觉总分 | 16.86 ± 0.75 | 17.05 ± 0.77 | 17.55 ± 0.77 | 19.11 ± 0.78 | F(3, 76) = 1.71 | 0.171 |

图3 实验结果。(A) tDCS对短时热痛疼痛强度的调控效应。(B) tDCS对短时热痛疼痛不愉悦度的调控效应。(C) tDCS对压痛阈限的调控效应。(D) lDLPFC+rM1-tDCS组各个被试中等疼痛强度评分变化值。(E) lDLPFC+rM1-tDCS组中等疼痛强度评分变化值与疼痛恐惧量表评分的相关分析。(F) lDLPFC+rM1-tDCS组中等疼痛强度评分变化值与疼痛灾难化量表评分的相关分析。 注:MP: 热刺激中等强度; HP: 热刺激高强度; lDLPFC+rM1: lDLPFC+rM1-tDCS组; lDLPFC: lDLPFC-tDCS组; rM1: rM1-tDCS组; * p < 0.05, ** p < 0.01; 数据呈现方式: 均值 ± 标准误。
| 变量 | 双靶点tDCS组 | 单靶点tDCS组 | 假刺激组 | 统计指标 | |
|---|---|---|---|---|---|
| F | p-fdr | ||||
| 状态焦虑量表 | 37.77 ± 1.64 | 36.46 ± 1.65 | 36.19 ± 1.79 | F(2, 50) = 1.25 | 0.294 |
| 负性情绪量表(后测−前测) | −0.81 ± 0.40 | 0.19 ± 0.62 | −0.39 ± 0.62 | F(2, 50) = 0.97 | 0.388 |
| 正性情绪量表(后测−前测) | −2.08 ± 0.45 | −1.73 ± 0.54 | −1.62 ± 0.96 | F(2, 50) = 0.17 | 0.845 |
| tDCS感觉总分 | 16.54 ± 0.68 | 16.35 ± 0.54 | 17.31 ± 0.57 | F(2, 50) = 1.58 | 0.215 |
表2 问卷统计结果(均值±标准误)
| 变量 | 双靶点tDCS组 | 单靶点tDCS组 | 假刺激组 | 统计指标 | |
|---|---|---|---|---|---|
| F | p-fdr | ||||
| 状态焦虑量表 | 37.77 ± 1.64 | 36.46 ± 1.65 | 36.19 ± 1.79 | F(2, 50) = 1.25 | 0.294 |
| 负性情绪量表(后测−前测) | −0.81 ± 0.40 | 0.19 ± 0.62 | −0.39 ± 0.62 | F(2, 50) = 0.97 | 0.388 |
| 正性情绪量表(后测−前测) | −2.08 ± 0.45 | −1.73 ± 0.54 | −1.62 ± 0.96 | F(2, 50) = 0.17 | 0.845 |
| tDCS感觉总分 | 16.54 ± 0.68 | 16.35 ± 0.54 | 17.31 ± 0.57 | F(2, 50) = 1.58 | 0.215 |
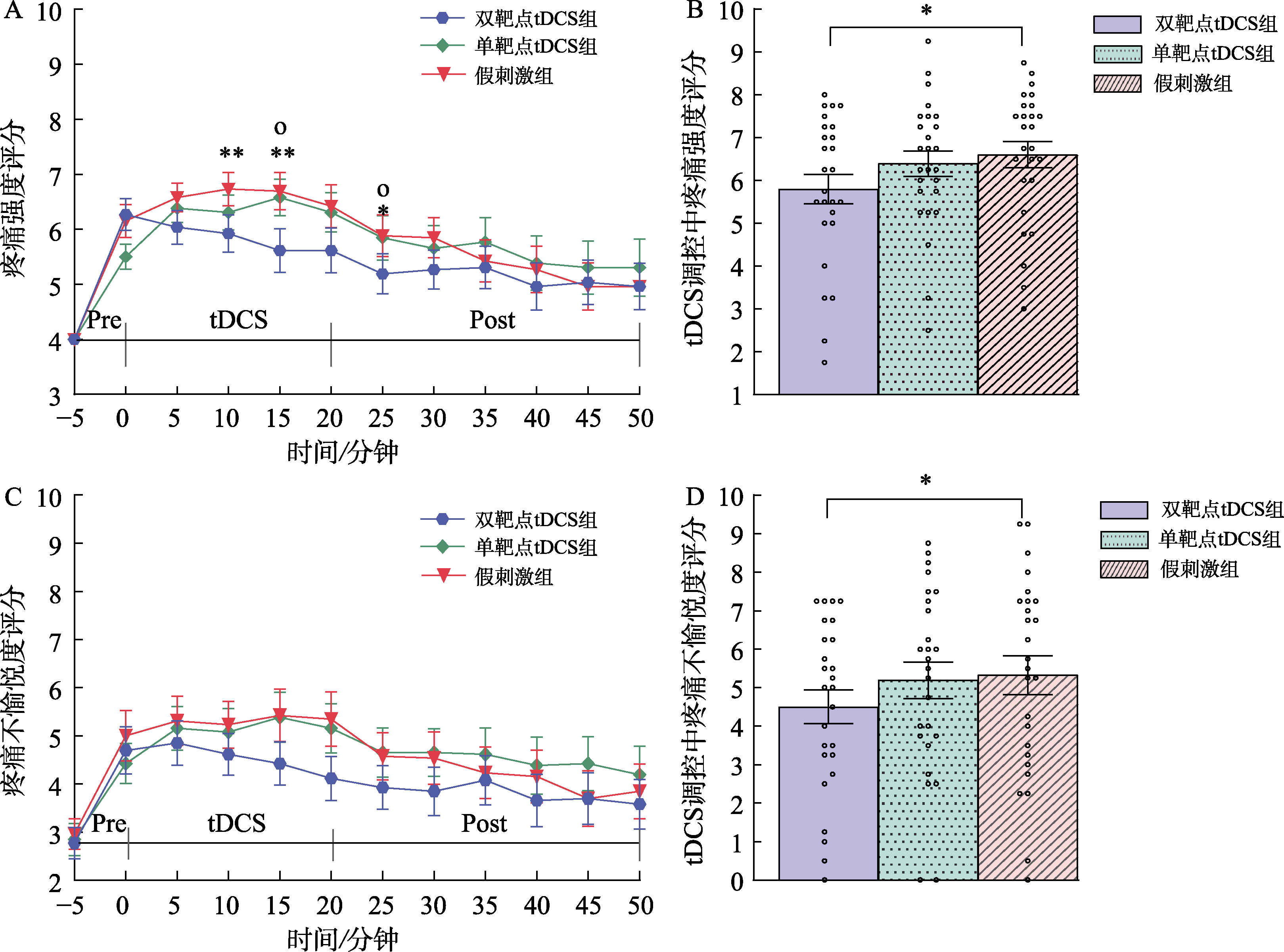
图4 tDCS对持续性疼痛的调控效应。(A) tDCS对持续性疼痛强度的调控效应。(B) tDCS对持续性疼痛不愉悦度的调控效应。(C) tDCS对调控过程中疼痛强度的调控效应。(D) tDCS对调控过程中疼痛不愉悦度的调控效应。 注: *表示成对比较后双靶点tDCS组与假刺激组之间存在显著性差异; ° 表示成对比较后双靶点tDCS组与单靶点tDCS组之间存在显著性差异; */° p < 0.05, ** p < 0.01; 数据呈现方式:均值 ± 标准误。
| 不痛 | 刚刚 感觉 到痛 | 最严重 的疼痛 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 想象你的小腿很重地撞在一个硬的边缘上, 例如:玻璃茶桌的边缘。你会觉得有多痛? | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2. 想象你的舌头被很热的饮料烫到。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 3. 想象你的肌肉因运动而有轻微的酸痛。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 4. 想象你的手指被抽屉夹到。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 5. 想象你用温水淋浴。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 6. 想象你的肩膀被轻度晒伤。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 7. 想象你从自行车上摔下擦伤了膝盖。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 8. 想象你吃东西的时候意外大力咬到舌头或脸颊。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 9. 想象你赤脚走过清凉的瓷砖地板。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 10. 想象你的手指被割了个小伤口, 而且无意中把盐撒到伤口上。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11. 想象月季花刺扎到你的手指尖。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 12. 想象你赤着双手拿冰袋几分钟。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 13. 想象你和一个正常手力的人握手。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 14. 想象你和一个手力很强的人握手。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 15. 想象你端起火锅时不小心抓到了跟锅一样发烫的锅耳。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 16. 想象你穿凉鞋时被穿厚重靴子的人踩到。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 17. 想象你手肘的麻筋撞到桌子的边缘。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 不痛 | 刚刚 感觉 到痛 | 最严重 的疼痛 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 想象你的小腿很重地撞在一个硬的边缘上, 例如:玻璃茶桌的边缘。你会觉得有多痛? | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2. 想象你的舌头被很热的饮料烫到。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 3. 想象你的肌肉因运动而有轻微的酸痛。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 4. 想象你的手指被抽屉夹到。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 5. 想象你用温水淋浴。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 6. 想象你的肩膀被轻度晒伤。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 7. 想象你从自行车上摔下擦伤了膝盖。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 8. 想象你吃东西的时候意外大力咬到舌头或脸颊。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 9. 想象你赤脚走过清凉的瓷砖地板。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 10. 想象你的手指被割了个小伤口, 而且无意中把盐撒到伤口上。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11. 想象月季花刺扎到你的手指尖。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 12. 想象你赤着双手拿冰袋几分钟。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 13. 想象你和一个正常手力的人握手。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 14. 想象你和一个手力很强的人握手。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 15. 想象你端起火锅时不小心抓到了跟锅一样发烫的锅耳。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 16. 想象你穿凉鞋时被穿厚重靴子的人踩到。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 17. 想象你手肘的麻筋撞到桌子的边缘。 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 完全没有 | 有些 | 中等程度 | 非常明显 | |
|---|---|---|---|---|
| 1. 我感到心情平静 | ① | ② | ③ | ④ |
| 2. 我感到安全 | ① | ② | ③ | ④ |
| 3. 我是紧张的 | ① | ② | ③ | ④ |
| 4. 我感到紧张束缚 | ① | ② | ③ | ④ |
| 5. 我感到安逸 | ① | ② | ③ | ④ |
| 6. 我感到烦乱 | ① | ② | ③ | ④ |
| 7. 我现在正烦恼, 感到这种烦恼超过了可能的不幸 | ① | ② | ③ | ④ |
| 8. 我感到满意 | ① | ② | ③ | ④ |
| 9. 我感到害怕 | ① | ② | ③ | ④ |
| 10. 我感到舒适 | ① | ② | ③ | ④ |
| 11. 我有自信心 | ① | ② | ③ | ④ |
| 12. 我觉得神经过敏 | ① | ② | ③ | ④ |
| 13. 我极度紧张不安 | ① | ② | ③ | ④ |
| 14. 我优柔寡断 | ① | ② | ③ | ④ |
| 15. 我是轻松的 | ① | ② | ③ | ④ |
| 16. 我感到心满意足 | ① | ② | ③ | ④ |
| 17. 我是烦恼的 | ① | ② | ③ | ④ |
| 18. 我感到慌乱 | ① | ② | ③ | ④ |
| 19. 我感觉镇定 | ① | ② | ③ | ④ |
| 20. 我感到愉快 | ① | ② | ③ | ④ |
| 完全没有 | 有些 | 中等程度 | 非常明显 | |
|---|---|---|---|---|
| 1. 我感到心情平静 | ① | ② | ③ | ④ |
| 2. 我感到安全 | ① | ② | ③ | ④ |
| 3. 我是紧张的 | ① | ② | ③ | ④ |
| 4. 我感到紧张束缚 | ① | ② | ③ | ④ |
| 5. 我感到安逸 | ① | ② | ③ | ④ |
| 6. 我感到烦乱 | ① | ② | ③ | ④ |
| 7. 我现在正烦恼, 感到这种烦恼超过了可能的不幸 | ① | ② | ③ | ④ |
| 8. 我感到满意 | ① | ② | ③ | ④ |
| 9. 我感到害怕 | ① | ② | ③ | ④ |
| 10. 我感到舒适 | ① | ② | ③ | ④ |
| 11. 我有自信心 | ① | ② | ③ | ④ |
| 12. 我觉得神经过敏 | ① | ② | ③ | ④ |
| 13. 我极度紧张不安 | ① | ② | ③ | ④ |
| 14. 我优柔寡断 | ① | ② | ③ | ④ |
| 15. 我是轻松的 | ① | ② | ③ | ④ |
| 16. 我感到心满意足 | ① | ② | ③ | ④ |
| 17. 我是烦恼的 | ① | ② | ③ | ④ |
| 18. 我感到慌乱 | ① | ② | ③ | ④ |
| 19. 我感觉镇定 | ① | ② | ③ | ④ |
| 20. 我感到愉快 | ① | ② | ③ | ④ |
| 题号 | 题目(PANAS) | 几乎没有 | 比较少 | 中等程度 | 比较多 | 极其多 |
|---|---|---|---|---|---|---|
| 1 | 感兴趣的 | 1 | 2 | 3 | 4 | 5 |
| 2 | 心烦的 | 1 | 2 | 3 | 4 | 5 |
| 3 | 精神活力高的 | 1 | 2 | 3 | 4 | 5 |
| 4 | 心神不宁的 | 1 | 2 | 3 | 4 | 5 |
| 5 | 劲头足的 | 1 | 2 | 3 | 4 | 5 |
| 6 | 内疚的 | 1 | 2 | 3 | 4 | 5 |
| 7 | 恐惧的 | 1 | 2 | 3 | 4 | 5 |
| 8 | 敌意的 | 1 | 2 | 3 | 4 | 5 |
| 9 | 热情的 | 1 | 2 | 3 | 4 | 5 |
| 10 | 自豪的 | 1 | 2 | 3 | 4 | 5 |
| 11 | 易怒的 | 1 | 2 | 3 | 4 | 5 |
| 12 | 警觉性高的 | 1 | 2 | 3 | 4 | 5 |
| 13 | 害羞的 | 1 | 2 | 3 | 4 | 5 |
| 14 | 备受鼓舞的 | 1 | 2 | 3 | 4 | 5 |
| 15 | 紧张的 | 1 | 2 | 3 | 4 | 5 |
| 16 | 意志坚定的 | 1 | 2 | 3 | 4 | 5 |
| 17 | 注意力集中的 | 1 | 2 | 3 | 4 | 5 |
| 18 | 坐立不安的 | 1 | 2 | 3 | 4 | 5 |
| 19 | 有活力的 | 1 | 2 | 3 | 4 | 5 |
| 20 | 害怕的 | 1 | 2 | 3 | 4 | 5 |
| 题号 | 题目(PANAS) | 几乎没有 | 比较少 | 中等程度 | 比较多 | 极其多 |
|---|---|---|---|---|---|---|
| 1 | 感兴趣的 | 1 | 2 | 3 | 4 | 5 |
| 2 | 心烦的 | 1 | 2 | 3 | 4 | 5 |
| 3 | 精神活力高的 | 1 | 2 | 3 | 4 | 5 |
| 4 | 心神不宁的 | 1 | 2 | 3 | 4 | 5 |
| 5 | 劲头足的 | 1 | 2 | 3 | 4 | 5 |
| 6 | 内疚的 | 1 | 2 | 3 | 4 | 5 |
| 7 | 恐惧的 | 1 | 2 | 3 | 4 | 5 |
| 8 | 敌意的 | 1 | 2 | 3 | 4 | 5 |
| 9 | 热情的 | 1 | 2 | 3 | 4 | 5 |
| 10 | 自豪的 | 1 | 2 | 3 | 4 | 5 |
| 11 | 易怒的 | 1 | 2 | 3 | 4 | 5 |
| 12 | 警觉性高的 | 1 | 2 | 3 | 4 | 5 |
| 13 | 害羞的 | 1 | 2 | 3 | 4 | 5 |
| 14 | 备受鼓舞的 | 1 | 2 | 3 | 4 | 5 |
| 15 | 紧张的 | 1 | 2 | 3 | 4 | 5 |
| 16 | 意志坚定的 | 1 | 2 | 3 | 4 | 5 |
| 17 | 注意力集中的 | 1 | 2 | 3 | 4 | 5 |
| 18 | 坐立不安的 | 1 | 2 | 3 | 4 | 5 |
| 19 | 有活力的 | 1 | 2 | 3 | 4 | 5 |
| 20 | 害怕的 | 1 | 2 | 3 | 4 | 5 |
| 你是否有以下症状或副作用? | 在下方填入体验的强度((1:从未有过; 2:轻微; 3:中度; 4:重度)) |
|---|---|
| 头痛 | |
| 颈部痛 | |
| 头皮痛 | |
| 刺痛 | |
| 瘙痒 | |
| 烧灼感 | |
| 皮肤发红 | |
| 困倦 | |
| 无法集中注意力 | |
| 急性心境变化 | |
| 光幻视 | |
| 其他(具体说明) |
| 你是否有以下症状或副作用? | 在下方填入体验的强度((1:从未有过; 2:轻微; 3:中度; 4:重度)) |
|---|---|
| 头痛 | |
| 颈部痛 | |
| 头皮痛 | |
| 刺痛 | |
| 瘙痒 | |
| 烧灼感 | |
| 皮肤发红 | |
| 困倦 | |
| 无法集中注意力 | |
| 急性心境变化 | |
| 光幻视 | |
| 其他(具体说明) |
| [1] |
Aslaksen P. M., Vasylenko O., & Fagerlund A. J. (2014). The effect of transcranial direct current stimulation on experimentally induced heat pain. Experimental Brain Research, 232(6), 1865-1873.
doi: 10.1007/s00221-014-3878-0 pmid: 24570387 |
| [2] | Benyamin R., Trescot A. M., Datta S., Buenaventura R. M., Adlaka R., Sehgal N., ... Vallejo R. (2008). Opioid complications and side effects. Pain Physician, 11(2S), S105-S120. |
| [3] |
Boggio P. S., Zaghi S., Lopes M., & Fregni F. (2008). Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. European Journal of Neurology, 15(10), 1124-1130.
doi: 10.1111/j.1468-1331.2008.02270.x pmid: 18717717 |
| [4] | Brighina F., De Tommaso M., Giglia F., Scalia S., Cosentino G., Puma A., ... Fierro B. (2011). Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. The Journal of Headache and Pain, 12(2), 185-191. |
| [5] |
Coghill R. C. (2020). The distributed nociceptive system: A framework for understanding pain. Trends in Neurosciences, 43(10), 780-794.
doi: S0166-2236(20)30168-5 pmid: 32800534 |
| [6] |
Compton W. M., Valentino R. J., & DuPont R. L. (2021). Polysubstance use in the U.S. opioid crisis. Molecular Psychiatry, 26(1), 41-50.
doi: 10.1038/s41380-020-00949-3 pmid: 33188253 |
| [7] |
Costa B., Ferreira I., Trevizol A., Thibaut A., & Fregni F. (2019). Emerging targets and uses of neuromodulation for pain. Expert Review of Neurotherapeutics, 19(2), 109-118.
doi: 10.1080/14737175.2019.1567332 pmid: 30681009 |
| [8] | Crombez G., Eccleston C., Van Damme S., Vlaeyen J. W., & Karoly P. (2012). Fear-avoidance model of chronic pain: The next generation. The Clinical Journal of Pain, 28(6), 475-483. |
| [9] | Cummiford C. M., Nascimento T. D., Foerster B. R., Clauw D. J., Zubieta J. K., Harris R. E., & DaSilva A. F. (2016). Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Research & Therapy, 18, 1-12. |
| [10] | DaSilva A. F., Mendonca M. E., Zaghi S., Lopes M., DosSantos M. F., Spierings E. L., ... Fregni F. (2012). TDCS-induced analgesia and electrical fields in pain- related neural networks in chronic migraine. Headache: The Journal of Head and Face Pain, 52(8), 1283-1295. |
| [11] | De Ridder D., Adhia D., & Vanneste S. (2021). The anatomy of pain and suffering in the brain and its clinical implications. Neuroscience & Biobehavioral Reviews, 130, 125-146. |
| [12] | Deer T. R., Krames E., Mekhail N., Pope J., Leong M., Stanton-Hicks M., ... Williams K. (2014). The appropriate use of neurostimulation: New and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation: Technology at the Neural Interface, 17(6), 599-615. |
| [13] | Deldar Z., Rustamov N., Bois S., Blanchette I., & Piche M. (2018). Enhancement of pain inhibition by working memory with anodal transcranial direct current stimulation of the left dorsolateral prefrontal cortex. The Journal of Physiological Sciences, 68(6), 825-836. |
| [14] |
DosSantos M. F., Love T. M., Martikainen I. K., Nascimento T. D., Fregni F., Cummiford C., ... Dasilva A. F. (2012). Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Frontiers in Psychiatry, 3, 93.
doi: 10.3389/fpsyt.2012.00093 pmid: 23130002 |
| [15] | DosSantos M. F., Martikainen I. K., Nascimento T. D., Love T. M., DeBoer M. D., Schambra H. M., ... DaSilva A. F. (2014). Building up analgesia in humans via the endogenous μ-opioid system by combining placebo and active tDCS: A preliminary report. PLoS One, 9(7), e102350. |
| [16] |
Fregni F., Boggio P. S., Lima M. C., Ferreira M. J., Wagner T., Rigonatti S. P., ... Pascual-Leone A. (2006). A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain, 122(1-2), 197-209.
doi: 10.1016/j.pain.2006.02.023 pmid: 16564618 |
| [17] | Garcia-Larrea L., & Peyron R. (2007). Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage, 37(Suppl. 1), S71-S79. |
| [18] | Garcı́a-Larrea L., Peyron R., Mertens P., Gregoire M., Lavenne F., Le Bars D., ... Laurent B. J. P. (1999). Electrical stimulation of motor cortex for pain control: A combined PET-scan and electrophysiological study. Pain, 83(2), 259-273. |
| [19] | George S. Z., Dannecker E. A., & Robinson M. E. (2006). Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: An experimental investigation in pain- free individuals. European Journal of Pain, 10(5), 457-465. |
| [20] | Goldberg D. S., & McGee S. J. (2011). Pain as a global public health priority. BMC Public Health, 11(1), 770. |
| [21] |
Gramsch C., Kattoor J., Icenhour A., Forsting M., Schedlowski M., Gizewski E. R., & Elsenbruch S. (2014). Learning pain-related fear: Neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiology of Learning and Memory, 116, 36-45.
doi: 10.1016/j.nlm.2014.08.003 pmid: 25128878 |
| [22] |
Gregoret L., Zamorano A. M., & Graven‐Nielsen T. (2021). Effects of multifocal transcranial direct current stimulation targeting the motor network during prolonged experimental pain. European Journal of Pain, 25(6), 1241-1253.
doi: 10.1002/ejp.1743 pmid: 33539582 |
| [23] | Gregoret L., Zamorano A. M., & Graven-Nielsen T. (2023). Multifocal tDCS targeting the motor network modulates event-related cortical responses during prolonged pain. The Journal of Pain, 24(2), 226-236. |
| [24] | Guo Z., Gong Y., Lu H., Qiu R., Wang X., Zhu X., & You X. (2022). Multitarget high-definition transcranial direct current stimulation improves response inhibition more than single-target high-definition transcranial direct current stimulation in healthy participants. Frontiers in Neuroscience, 16, 905247. |
| [25] | Gurdiel-Álvarez F., González-Zamorano Y., Lerma Lara S., Gómez-Soriano J., Taylor J., Romero J. P., ... Fernández- Carnero J. (2021). Effectiveness of unihemispheric concurrent dual-site stimulation over M1 and dorsolateral prefrontal cortex stimulation on pain processing: A triple blind cross-over control trial. Brain Sciences, 11(2), 188. |
| [26] | Hirsh A. T., George S. Z., Bialosky J. E., & Robinson M. E. (2008). Fear of pain, pain catastrophizing, and acute pain perception: Relative prediction and timing of assessment. The Journal of Pain, 9(9), 806-812. |
| [27] | Icenhour A., Langhorst J., Benson S., Schlamann M., Hampel S., Engler H., ... Elsenbruch S. (2015). Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterology & Motility, 27(1), 114-127. |
| [28] |
Jiang N., Wei J., Li G., Wei B., Zhu F. F., & Hu Y. (2020). Effect of dry-electrode-based transcranial direct current stimulation on chronic low back pain and low back muscle activities: A double-blind sham-controlled study. Restorative Neurology and Neuroscience, 38(1), 41-54.
doi: 10.3233/RNN-190922 pmid: 31683491 |
| [29] | Jiang X., Wang Y., Wan R., Feng B., Zhang Z., Lin Y., & Wang Y. (2022). The effect of high-definition transcranial direct current stimulation on pain processing in a healthy population: A single-blinded crossover controlled study. Neuroscience Letters, 767, 136304. |
| [30] |
Jürgens T. P., Schulte A., Klein T., & May A. (2012). Transcranial direct current stimulation does neither modulate results of a quantitative sensory testing protocol nor ratings of suprathreshold heat stimuli in healthy volunteers. European Journal of Pain, 16(9), 1251-1263.
doi: 10.1002/j.1532-2149.2012.00135.x pmid: 22416036 |
| [31] |
Khedr E. M., Omran E. A., Ismail N. M., El-Hammady D. H., Goma S. H., Kotb H., ... Ahmed G. A. (2017). Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimulation, 10(5), 893-901.
doi: S1935-861X(17)30838-0 pmid: 28684258 |
| [32] |
Kikkert S., Mezue M., O'Shea J., Henderson Slater D., Johansen-Berg H., Tracey I., & Makin T. R. (2019). Neural basis of induced phantom limb pain relief. Annals of neurology, 85(1), 59-73.
doi: 10.1002/ana.25371 pmid: 30383312 |
| [33] | Knotkova H., Hamani C., Sivanesan E., Le Beuffe M. F. E., Moon J. Y., Cohen S. P., & Huntoon M. A. (2021). Neuromodulation for chronic pain. The Lancet, 397(10289), 2111-2124. |
| [34] |
Knotkova H., Nitsche M. A., & Cruciani R. A. (2013). Putative physiological mechanisms underlying tDCS analgesic effects. Frontiers in Human Neuroscience, 7, 628.
doi: 10.3389/fnhum.2013.00628 pmid: 24133434 |
| [35] | Kold S., & Graven-Nielsen T. (2021). Effect of anodal high- definition transcranial direct current stimulation on the pain sensitivity in a healthy population: A double-blind, sham- controlled study. Pain, 162(6), 1659-1668. |
| [36] | Krames E. S., Peckham P. H., Rezai A., & Aboelsaad F. (2009). What is neuromodulation? Elliot S. Krames, P. Hunter Peckham, Ali R. Rezai, Neuromodulation (pp. 3-8). Academic Press. |
| [37] | Kucyi A., Salomons T. V., & Davis K. D. (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences, 110(46), 18692-18697. |
| [38] | Lefaucheur J. P., Antal A., Ayache S. S., Benninger D. H., Brunelin J., Cogiamanian F., ... Paulus W. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 128(1), 56-92. |
| [39] | Li Z. J., Zhang L. B., Chen Y. X., & Hu L. (2023). Advancements and challenges in neuromodulation technology: Interdisciplinary opportunities and collaborative endeavors. Science Bulletin, 68(18), 1978-1982. |
| [40] | Lin R. L., Douaud G., Filippini N., Okell T. W., Stagg C. J., & Tracey I. (2017). Structural connectivity variances underlie functional and behavioral changes during pain relief induced by neuromodulation. Scientific Reports, 7(1), 41603. |
| [41] | Lloyd D. M., Wittkopf P. G., Arendsen L. J., & Jones A. K. (2020). Is transcranial direct current stimulation (tDCS) effective for the treatment of pain in fibromyalgia? A systematic review and meta-analysis. The Journal of Pain, 21(11-12), 1085-1100. |
| [42] | Luedtke K., Rushton A., Wright C., Jürgens T., Polzer A., Mueller G., & May A. (2015). Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: Sham controlled double blinded randomised controlled trial. British Medical Journal, 350, h1640. |
| [43] |
Manor B., Dagan M., Herman T., Gouskova N. A., Vanderhorst V. G., Giladi N., ... Hausdorff J. M. (2021). Multitarget transcranial electrical stimulation for freezing of gait: A randomized controlled trial. Movement Disorders, 36(11), 2693-2698.
doi: 10.1002/mds.28759 pmid: 34406695 |
| [44] |
Markfelder T., & Pauli P. (2020). Fear of pain and pain intensity: Meta-analysis and systematic review. Psychological Bulletin, 146(5), 411-450.
doi: 10.1037/bul0000228 pmid: 32212745 |
| [45] | Mori F., Codecà C., Kusayanagi H., Monteleone F., Buttari F., Fiore S., ... Centonze D. (2010). Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. The Journal of Pain, 11(5), 436-442. |
| [46] |
Pagano R. L., Fonoff E. T., Dale C. S., Ballester G., Teixeira M. J., & Britto L. R. (2012). Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: Possible pathways for antinociception. Pain, 153(12), 2359-2369.
doi: 10.1016/j.pain.2012.08.002 pmid: 23017297 |
| [47] |
Palm U., Reisinger E., Keeser D., Kuo M. F., Pogarell O., Leicht G., ... Padberg F. (2013). Evaluation of sham transcranial direct current stimulation for randomized, placebo-controlled clinical trials. Brain Stimulation, 6(4), 690-695.
doi: 10.1016/j.brs.2013.01.005 pmid: 23415938 |
| [48] |
Peltz E., Seifert F., DeCol R., Dörfler A., Schwab S., & Maihöfner C. (2011). Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage, 54(2), 1324-1335.
doi: 10.1016/j.neuroimage.2010.09.012 pmid: 20851770 |
| [49] |
Peyron R., Faillenot I., Mertens P., Laurent B., & Garcia- Larrea L. (2007). Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage, 34(1), 310-321.
doi: 10.1016/j.neuroimage.2006.08.037 pmid: 17055297 |
| [50] |
Peyron R., Garcia-Larrea L., Deiber M. P., Cinotti L., Convers P., Sindou M., ... Laurent B. (1995). Electrical stimulation of precentral cortical area in the treatment of central pain: Electrophysiological and PET study. Pain, 62(3), 275-286.
doi: 10.1016/0304-3959(94)00211-V pmid: 8657427 |
| [51] | Peyron R., Laurent B., & García-Larrea L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiologie Clinique/Clinical Neurophysiology, 30(5), 263-288. |
| [52] |
Przeklasa-Muszyńska A., Kocot-Kępska M., Dobrogowski J., Wiatr M., & Mika J. (2017). Transcranial direct current stimulation (tDCS) and its influence on analgesics effectiveness in patients suffering from migraine headache. Pharmacological Reports, 69(4), 714-721.
doi: S1734-1140(16)30461-3 pmid: 28551531 |
| [53] | Reidler J. S., Mendonca M. E., Santana M. B., Wang X., Lenkinski R., Motta A. F., ... Fregni F. (2012). Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. The Journal of Pain, 13(5), 450-458. |
| [54] |
Rice A. S., Smith B. H., & Blyth F. M. (2016). Pain and the global burden of disease. Pain, 157(4), 791-796.
doi: 10.1097/j.pain.0000000000000454 pmid: 26670465 |
| [55] | Sakas D. E., Panourias I. G., Simpson B. A., & Krames E. S. (2007). An introduction to operative neuromodulation and functional neuroprosthetics, the new frontiers of clinical neuroscience and biotechnology. Acta Neurochirurgica, 97(Pt 1), 3-10. |
| [56] | Schmidt-Wilcke T. (2015). Neuroimaging of chronic pain. Best Practice & Research Clinical Rheumatology, 29(1), 29-41. |
| [57] | Seminowicz D. A., & Moayedi M. (2017). The dorsolateral prefrontal cortex in acute and chronic pain. The Journal of Pain, 18(9), 1027-1035. |
| [58] | Staahl C., & Drewes A. M. (2004). Experimental human pain models: A review of standardised methods for preclinical testing of analgesics. Basic & clinical pharmacology & toxicology, 95(3), 97-111. |
| [59] |
Stamenkovic D. M., Mladenovic K., Rancic N., Cvijanovic V., Maric N., Neskovic V., ... Ilic T. V. (2020). Effect of transcranial direct current stimulation combined with patient-controlled intravenous morphine analgesia on analgesic use and post-thoracotomy pain. A prospective, randomized, double-blind, sham-controlled, proof-of-concept clinical trial. Frontiers in Pharmacology, 11, 125.
doi: 10.3389/fphar.2020.00125 pmid: 32161547 |
| [60] |
Taylor J. J., Borckardt J. J., & George M. S. (2012). Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain, 153(6), 1219-1225.
doi: 10.1016/j.pain.2012.02.030 pmid: 22444187 |
| [61] | Tu Y., Wilson G., Camprodon J., Dougherty D. D., Vangel M., Benedetti F., ... Kong J. (2021). Manipulating placebo analgesia and nocebo hyperalgesia by changing brain excitability. Proceedings of the National Academy of Sciences, 118(19), e2101273118. |
| [62] |
Valle A., Roizenblatt S., Botte S., Zaghi S., Riberto M., Tufik S., ... Fregni F. (2009). Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. Journal of Pain Management, 2(3), 353-361.
pmid: 21170277 |
| [63] |
Vaseghi B., Zoghi M., & Jaberzadeh S. (2014). Does anodal transcranial direct current stimulation modulate sensory perception and pain? A meta-analysis study. Clinical Neurophysiology, 125(9), 1847-1858.
doi: 10.1016/j.clinph.2014.01.020 pmid: 24555922 |
| [64] | Vaseghi B., Zoghi M., & Jaberzadeh S. (2015a). The effects of anodal-tDCS on corticospinal excitability enhancement and its after-effects: Conventional vs. unihemispheric concurrent dual-site stimulation. Frontiers in Human Neuroscience, 9, 533. |
| [65] | Vaseghi B., Zoghi M., & Jaberzadeh S. (2015b). How does anodal transcranial direct current stimulation of the pain neuromatrix affect brain excitability and pain perception? A randomised, double-blind, sham-control study. PloS One, 10(3), e0118340. |
| [66] |
Volders S., Boddez Y., De Peuter S., Meulders A., & Vlaeyen J. W. (2015). Avoidance behavior in chronic pain research: A cold case revisited. Behaviour Research and Therapy, 64, 31-37.
doi: 10.1016/j.brat.2014.11.003 pmid: 25506905 |
| [67] | Wen Y. R., Shi J., Hu Z. Y., Lin Y. Y., Lin Y. T., Jiang X., ... Wang Y. L. (2022). Is transcranial direct current stimulation beneficial for treating pain, depression, and anxiety symptoms in patients with chronic pain? A systematic review and meta-analysis. Frontiers in Molecular Neuroscience, 15, 1056966. |
| [68] |
Yu S., Liu R., Zhao G., Yang X., Qiao X., Feng J., ... Steiner T. (2012). The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache, 52(4), 582-591.
pmid: 22590713 |
| [69] | Zandieh A., Parhizgar S. E., Fakhri M., Taghvaei M., Miri S., Shahbabaie A., ... Ekhtiari H. (2013). Modulation of cold pain perception by transcranial direct current stimulation in healthy individuals. Neuromodulation: Technology at the Neural Interface, 16(4), 345-348. |
| [70] | Zhang F., Xiang W., Li C. Y., & Li S. C. (2016). Economic burden of irritable bowel syndrome in China. World Journal of Gastroenterology, 22(47), 10450. |
| [71] |
Zhou J., Manor B., Yu W., Lo O. Y., Gouskova N., Salvador R., ... Hausdorff J. M. (2021). Targeted tDCS mitigates dual-task costs to gait and balance in older adults. Annals of Neurology, 90(3), 428-439.
doi: 10.1002/ana.26156 pmid: 34216034 |
| [1] | 高可翔, 张岳瑶, 李思瑾, 袁加锦, 李红, 张丹丹. 腹内侧前额叶在内隐认知重评中的因果作用[J]. 心理学报, 2023, 55(2): 210-223. |
| [2] | 华艳, 李明霞, 王巧婷, 冯彩霞, 张晶. 左侧眶额皮层在自动情绪调节下注意选择中的作用:来自经颅直流电刺激的证据[J]. 心理学报, 2020, 52(9): 1048-1056. |
| [3] | 曹娜, 孟海江, 王艳秋, 邱方晖, 谭晓缨, 吴殷, 张剑. 左侧背外侧前额叶在程序性运动学习中的作用[J]. 心理学报, 2020, 52(5): 597-608. |
| [4] | 殷西乐, 李建标, 陈思宇, 刘晓丽, 郝洁. 第三方惩罚的神经机制:来自经颅直流电刺激的证据[J]. 心理学报, 2019, 51(5): 571-583. |
| [5] | 张丹丹, 刘珍莉, 陈钰, 买晓琴. 右腹外侧前额叶对高抑郁水平成年人社会情绪调节的作用:一项tDCS研究[J]. 心理学报, 2019, 51(2): 207-2015. |
| [6] | 王思思, 库逸轩. 右侧背外侧前额叶在视觉工作记忆中的因果性作用[J]. 心理学报, 2018, 50(7): 727-738. |
| [7] | 王慧慧, 罗玉丹, 石冰, 余凤琼, 汪凯. 经颅直流电刺激对健康大学生反应抑制的影响 *[J]. 心理学报, 2018, 50(6): 647-654. |
| [8] | 甘甜, 石睿, 刘超, 罗跃嘉. 经颅直流电刺激右侧颞顶联合区 对助人意图加工的影响[J]. 心理学报, 2018, 50(1): 36-46. |
| [9] | 罗俊; 叶航;郑昊力;贾拥民;陈姝; 黄达强. 左右侧颞顶联合区对道德意图信息加工能力的共同作用——基于经颅直流电刺激技术[J]. 心理学报, 2017, 49(2): 228-240. |
| [10] | 窦伟伟;郑希付;杨慧芳;王俊芳;李悦;俄小天;陈倩倩. 认知分心的强度对创伤性信息加工的影响[J]. 心理学报, 2014, 46(5): 656-665. |
| [11] | 甘甜;李万清;唐红红;陆夏平;李小俚;刘超;罗跃嘉. 经颅直流电刺激右侧颞顶联合区对道德意图加工的影响[J]. 心理学报, 2013, 45(9): 1004-1014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||