

CN 11-4766/R
主办:中国科学院心理研究所
出版:科学出版社


心理科学进展 ›› 2023, Vol. 31 ›› Issue (3): 402-415.doi: 10.3724/SP.J.1042.2023.00402 cstr: 32111.14.2023.00402
收稿日期:2021-10-15
出版日期:2023-03-15
发布日期:2022-12-22
通讯作者:
雷怡, E-mail: 642034842@qq.com基金资助:
LIU Peihan1, ZHANG Huoyin2, ZHANG Xukai1, LI Hong1, LEI Yi1( )
)
Received:2021-10-15
Online:2023-03-15
Published:2022-12-22
摘要:
疼痛和奖赏能够为个体提供不同的行为动机和主观价值体验, 寻求奖赏和避免疼痛对于生存都很重要。疼痛可划分为急性疼痛和慢性疼痛, 奖赏可区分为预期阶段的动机成分和体验阶段的享乐成分。奖赏对疼痛的抑制作用已经被广泛证实, 但关于疼痛对奖赏的影响, 目前的研究结果并不一致。因此需要进一步区分并探究急性疼痛与慢性疼痛对奖赏加工不同阶段的影响, 分析两种疼痛对奖赏加工产生不一致影响的现象。这种现象出现的原因可能与急性疼痛向慢性疼痛转变过程中出现的奖赏加工能力缺陷有关。未来可以考虑从改善奖赏加工能力缺陷的角度进行检测和治疗, 提前预防急性疼痛向慢性疼痛转变。
中图分类号:
刘沛菡, 张火垠, 张旭凯, 李红, 雷怡. (2023). 急性疼痛与慢性疼痛对奖赏加工的影响及神经机制. 心理科学进展 , 31(3), 402-415.
LIU Peihan, ZHANG Huoyin, ZHANG Xukai, LI Hong, LEI Yi. (2023). Effects of acute versus chronic pain on reward processing and the underlying neural mechanisms involved. Advances in Psychological Science, 31(3), 402-415.
| 研究 | 被试类型 | 刺激类型 | 奖赏任务/刺激 | 奖赏成分 | 研究方法 | 指标 |
|---|---|---|---|---|---|---|
| Zubieta, | 人类 | 实验室诱导急性疼痛 | - | 阿片系统与享乐成分相关 | PET实验 | 口腔面部表情 |
| Foo et al., | 大鼠 | 注射福尔马林 | 蔗糖奖赏 | 动机成分及 享乐成分无变化 | - | 进食行为的差异 |
| Low & Fitzgerald, | 大鼠 | 皮肤切口 | 蔗糖奖赏 | 动机成分  | - | 感觉敏感性 |
| Darbor et al., | 人类 | 冷压刺激 | 食物奖赏 | 动机成分  | 行为实验 | 疼痛强度评分 食物重量消耗 |
| Gandhi et al., | 人类 | 热痛刺激 | MID任务 (金钱奖赏) | 动机成分  | 行为实验 | RT、SCR 疼痛强度、不愉快程度评分 |
| Becker et al., | 人类 | 热痛刺激 | 金钱决策任务 (金钱奖赏) | 享乐成分  | 行为实验 | 奖赏喜爱程度评分 |
| Wang et al., | 人类 | 热痛刺激 (辣椒素) | 金钱决策任务 (金钱奖赏) | 动机成分  | 行为实验 | 疼痛情感评分 阿片系统的激活 |
| Wang et al., | 人类 | 热痛刺激 (辣椒素) | 猜牌游戏 (金钱奖赏) | 享乐成分  | fMRI实验 | 主观幸福感评分 |
表1 急性疼痛对奖赏加工的影响
| 研究 | 被试类型 | 刺激类型 | 奖赏任务/刺激 | 奖赏成分 | 研究方法 | 指标 |
|---|---|---|---|---|---|---|
| Zubieta, | 人类 | 实验室诱导急性疼痛 | - | 阿片系统与享乐成分相关 | PET实验 | 口腔面部表情 |
| Foo et al., | 大鼠 | 注射福尔马林 | 蔗糖奖赏 | 动机成分及 享乐成分无变化 | - | 进食行为的差异 |
| Low & Fitzgerald, | 大鼠 | 皮肤切口 | 蔗糖奖赏 | 动机成分  | - | 感觉敏感性 |
| Darbor et al., | 人类 | 冷压刺激 | 食物奖赏 | 动机成分  | 行为实验 | 疼痛强度评分 食物重量消耗 |
| Gandhi et al., | 人类 | 热痛刺激 | MID任务 (金钱奖赏) | 动机成分  | 行为实验 | RT、SCR 疼痛强度、不愉快程度评分 |
| Becker et al., | 人类 | 热痛刺激 | 金钱决策任务 (金钱奖赏) | 享乐成分  | 行为实验 | 奖赏喜爱程度评分 |
| Wang et al., | 人类 | 热痛刺激 (辣椒素) | 金钱决策任务 (金钱奖赏) | 动机成分  | 行为实验 | 疼痛情感评分 阿片系统的激活 |
| Wang et al., | 人类 | 热痛刺激 (辣椒素) | 猜牌游戏 (金钱奖赏) | 享乐成分  | fMRI实验 | 主观幸福感评分 |
| 研究 | 被试类型 | 刺激类型 | 奖赏任务/刺激 | 奖励成分 | 研究方法 | 指标 |
|---|---|---|---|---|---|---|
| Marbach et al., | 人类 | CLBP | - | 享乐成分  | 行为实验 | 身体快感缺失量表 |
| Becerra-García & Robles Jurado, | 人类 | FM | - | 动机成分  | 行为实验 | 行为方法系统的问卷 |
| Y.-T. Liu et al., | 小鼠 | CWP | 蔗糖奖赏 | 动机成分  享乐成分  | - | 蔗糖奖赏消耗模式 |
| Okun et al., | 大鼠 | 术后神经病理性疼痛 | 蔗糖奖赏 | 动机成分及 享乐成分无变化 | - | 口腔面部表情 |
| Schwartz et al., | 小鼠 | 慢性炎症性/神经病理性疼痛 | 蔗糖奖赏 | 动机成分 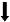 享乐成分 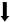 | - | 蔗糖奖赏消耗模式 |
| Salcido et al., | 小鼠 | 慢性炎症性疼痛 | 接近-回避范式 (蔗糖奖赏) | 动机成分 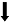 | - | 杠杆按压次数 |
| Small & Apkarian, | 人类 | CBP | 食物奖赏 | 享乐成分无变化 | 行为实验 | 奖赏喜爱程度评分 |
| Geha et al., | 人类 | CLBP | 食物奖赏 | 享乐成分 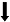 | 行为实验 | 奖赏喜爱程度评分 |
| Nees et al., | 人类 | CBP | 社会奖赏 | 享乐成分  | fMRI实验 | 奖赏喜爱程度评分 |
| Kocselet al., | 人类 | EM | MID任务 (金钱奖赏) | 享乐成分  | fMRI实验 | 奖赏喜爱程度评分 |
| Martucciet al., | 人类 | FM | MID任务 (金钱奖赏) | 动机成分  | fMRI实验 | RT 奖赏唤醒度评分 |
| Kim et al., | 人类 | FM CLBP | MID任务 (金钱奖赏) | 动机成分  享乐成分  | fMRI实验 | RT 快感缺失及BDI评分 |
表2 慢性疼痛对奖赏加工的影响
| 研究 | 被试类型 | 刺激类型 | 奖赏任务/刺激 | 奖励成分 | 研究方法 | 指标 |
|---|---|---|---|---|---|---|
| Marbach et al., | 人类 | CLBP | - | 享乐成分  | 行为实验 | 身体快感缺失量表 |
| Becerra-García & Robles Jurado, | 人类 | FM | - | 动机成分  | 行为实验 | 行为方法系统的问卷 |
| Y.-T. Liu et al., | 小鼠 | CWP | 蔗糖奖赏 | 动机成分  享乐成分  | - | 蔗糖奖赏消耗模式 |
| Okun et al., | 大鼠 | 术后神经病理性疼痛 | 蔗糖奖赏 | 动机成分及 享乐成分无变化 | - | 口腔面部表情 |
| Schwartz et al., | 小鼠 | 慢性炎症性/神经病理性疼痛 | 蔗糖奖赏 | 动机成分  享乐成分  | - | 蔗糖奖赏消耗模式 |
| Salcido et al., | 小鼠 | 慢性炎症性疼痛 | 接近-回避范式 (蔗糖奖赏) | 动机成分  | - | 杠杆按压次数 |
| Small & Apkarian, | 人类 | CBP | 食物奖赏 | 享乐成分无变化 | 行为实验 | 奖赏喜爱程度评分 |
| Geha et al., | 人类 | CLBP | 食物奖赏 | 享乐成分  | 行为实验 | 奖赏喜爱程度评分 |
| Nees et al., | 人类 | CBP | 社会奖赏 | 享乐成分  | fMRI实验 | 奖赏喜爱程度评分 |
| Kocselet al., | 人类 | EM | MID任务 (金钱奖赏) | 享乐成分  | fMRI实验 | 奖赏喜爱程度评分 |
| Martucciet al., | 人类 | FM | MID任务 (金钱奖赏) | 动机成分  | fMRI实验 | RT 奖赏唤醒度评分 |
| Kim et al., | 人类 | FM CLBP | MID任务 (金钱奖赏) | 动机成分  享乐成分  | fMRI实验 | RT 快感缺失及BDI评分 |
| [1] | 陈乐乐, 黄蓉, 贾世伟. (2020). 反馈相关负波与成瘾. 心理科学进展, 28(6), 959-969. |
| [2] | 李丹阳, 李鹏, 李红. (2018). 反馈负波及其近10年理论解释. 心理科学进展, 26(9), 1642-1650. |
| [3] | 李琪, 许晶, 郑亚. (2017). 刺激前负波:奖赏期待的电生理指标. 心理科学进展, 25(7), 1114-1121. |
| [4] |
Admon, R., & Pizzagalli, D. A. (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114-118.
pmid: 26258159 |
| [5] |
Apkarian, V. A., Sosa, Y., Krauss, B. R., Thomas, S. P., Fredrickson, B. E., Levy, R. E., Harden, N. R., & Chialvo, D. R. (2004). Chronic pain patients are impaired on an emotional decision-making task. Pain, 108(1-2), 129-136.
doi: 10.1016/j.pain.2003.12.015 pmid: 15109516 |
| [6] |
Baliki, M. N., & Apkarian, A. V. (2015). Nociception, pain, negative moods, and behavior selection. Neuron, 87(3), 474-491.
doi: 10.1016/j.neuron.2015.06.005 pmid: 26247858 |
| [7] |
Baliki, M. N., Petre, B., Torbey, S., Herrmann, K. M., Huang, L., Schnitzer, T. J., Fields, H. L., & Apkarian, A. V. (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nature Neuroscience, 15(8), 1117-1119.
doi: 10.1038/nn.3153 pmid: 22751038 |
| [8] |
Becerra-García, J. A., & Robles Jurado, M. J. (2014). Behavioral approach system activity and self-reported somatic symptoms in fibromyalgia: An exploratory study. International Journal of Rheumatic Diseases, 17(1), 89-92.
doi: 10.1111/1756-185X.12034 pmid: 24472271 |
| [9] |
Becker, S., Gandhi, W., Chen, Y. J., & Schweinhardt, P. (2017). Subjective utility moderates bidirectional effects of conflicting motivations on pain perception. Scientific Reports, 7(1), 7790.
doi: 10.1038/s41598-017-08454-4 pmid: 28798478 |
| [10] |
Becker, S., Gandhi, W., Elfassy, N. M., & Schweinhardt, P. (2013). The role of dopamine in the perceptual modulation of nociceptive stimuli by monetary wins or losses. European Journal of Neuroscience, 38(7), 3080-3088.
doi: 10.1111/ejn.12303 pmid: 23841460 |
| [11] |
Becker, S., Gandhi, W., & Schweinhardt, P. (2012). Cerebral interactions of pain and reward and their relevance for chronic pain. Neuroscience Letters, 520(2), 182-187.
doi: 10.1016/j.neulet.2012.03.013 pmid: 22440855 |
| [12] |
Becker, S., Kleinböhl, D., Baus, D., & Hölzl, R. (2011). Operant learning of perceptual sensitization and habituation is impaired in fibromyalgia patients with and without irritable bowel syndrome. Pain, 152(6), 1408-1417.
doi: 10.1016/j.pain.2011.02.027 pmid: 21439728 |
| [13] |
Becker, S., Löffler, M., & Seymour, B. (2020). Reward enhances pain discrimination in humans. Psychological Science, 31(9), 1191-1199.
doi: 10.1177/0956797620939588 pmid: 32818387 |
| [14] |
Berridge, K. C., & Kringelbach, M. L. (2015). Pleasure systems in the brain. Neuron, 86(3), 646-664.
doi: 10.1016/j.neuron.2015.02.018 pmid: 25950633 |
| [15] |
Berridge, K. C., Robinson, T. E., & Aldridge, J. W. (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65-73.
doi: 10.1016/j.coph.2008.12.014 pmid: 19162544 |
| [16] |
Bromberg-Martin, E. S., Matsumoto, M., & Hikosaka, O. (2010). Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron, 68(5), 815-834.
doi: 10.1016/j.neuron.2010.11.022 pmid: 21144997 |
| [17] |
Bushnell, M. C., Čeko, M., & Low, L. A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews Neuroscience, 14(7), 502-511.
doi: 10.1038/nrn3516 pmid: 23719569 |
| [18] |
Cameron, J. D., Goldfield, G. S., Finlayson, G., Blundell, J. E., & Doucet, É. (2014). Fasting for 24 hours heightens reward from food and food-related cues. PLoS ONE, 9(1), e85970.
doi: 10.1371/journal.pone.0085970 URL |
| [19] |
Chapman, C. R., & Vierck, C. J. (2017). The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. The Journal of Pain, 18(4), 359.e1-359.e38.
doi: 10.1016/j.jpain.2016.11.004 URL |
| [20] |
Cowen, S. L., Phelps, C. E., Navratilova, E., McKinzie, D. L., Okun, A., Husain, O., Gleason, S. D., Witkin, J. M., & Porreca, F. (2018). Chronic pain impairs cognitive flexibility and engages novel learning strategies in rats. Pain, 159(7), 1403-1412.
doi: 10.1097/j.pain.0000000000001226 pmid: 29578947 |
| [21] |
Darbor, K. E., Lench, H. C., & Carter-Sowell, A. R. (2016). Do people eat the pain away? The effects of acute physical pain on subsequent consumption of sweet-tasting food. PLOS ONE, 11(11), e0166931.
doi: 10.1371/journal.pone.0166931 URL |
| [22] |
DosSantos, M. F., de Souza Moura, B., & DaSilva, A. F. (2017). Reward circuitry plasticity in pain perception and modulation. Frontiers in Pharmacology, 8, 790.
doi: 10.3389/fphar.2017.00790 pmid: 29209204 |
| [23] |
Eisenberger, N. I. (2012). The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience, 13(6), 421-434.
doi: 10.1038/nrn3231 pmid: 22551663 |
| [24] |
Etkin, A., Egner, T., & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85-93.
doi: 10.1016/j.tics.2010.11.004 pmid: 21167765 |
| [25] |
Fields, H. (2004). State-dependent opioid control of pain. Nature Reviews Neuroscience, 5(7), 565-575.
doi: 10.1038/nrn1431 pmid: 15208698 |
| [26] |
Fields, H. (2007). Understanding how opioids contribute to reward and analgesia. Regional Anesthesia and Pain Medicine, 32(3), 242-246.
pmid: 17543821 |
| [27] |
Finan, P. H., Letzen, J., Epstein, D. H., Mun, C. J., Stull, S., Kowalczyk, W. J., ... Preston, K. L. (2021). Reward responsiveness in patients with opioid use disorder on opioid agonist treatment: Role of comorbid chronic pain. Pain Medicine, 22(9), 2019-2027.
doi: 10.1093/pm/pnab031 pmid: 33624802 |
| [28] |
Foo, H., Crabtree, K., Thrasher, A., & Mason, P. (2009). Eating is a protected behavior even in the face of persistent pain in male rats. Physiology & Behavior, 97(3-4), 426-429.
doi: 10.1016/j.physbeh.2009.03.015 URL |
| [29] |
Gandhi, W., Becker, S., & Schweinhardt, P. (2013). Pain increases motivational drive to obtain reward, but does not affect associated hedonic responses: A behavioural study in healthy volunteers. European Journal of Pain, 17(7), 1093-1103.
doi: 10.1002/j.1532-2149.2012.00281.x pmid: 23349058 |
| [30] |
Geha, P., deAraujo, I., Green, B., & Small, D. M. (2014). Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain, 155(4), 712-722.
doi: 10.1016/j.pain.2013.12.027 pmid: 24384160 |
| [31] |
Glare, P., Aubrey, K. R., & Myles, P. S. (2019). Transition from acute to chronic pain after surgery. The Lancet, 393(10180), 1537-1546.
doi: 10.1016/S0140-6736(19)30352-6 URL |
| [32] |
Gureje, O., von Korff, M., Kola, L., Demyttenaere, K., He, Y., Posada-Villa, J., ... Alonso, J. (2008). The relation between multiple pains and mental disorders: Results from the World Mental Health Surveys. Pain, 135(1-2), 82-91.
doi: 10.1016/j.pain.2007.05.005 pmid: 17570586 |
| [33] |
Haack, M., Simpson, N., Sethna, N., Kaur, S., & Mullington, J. (2020). Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology, 45(1), 205-216.
doi: 10.1038/s41386-019-0439-z pmid: 31207606 |
| [34] |
Harris, R. E., Clauw, D. J., Scott, D. J., McLean, S. A., Gracely, R. H., & Zubieta, J.-K. (2007). Decreased central μ-opioid receptor availability in fibromyalgia. Journal of Neuroscience, 27(37), 10000-10006.
doi: 10.1523/JNEUROSCI.2849-07.2007 URL |
| [35] |
Hashmi, J. A., Baliki, M. N., Huang, L., Baria, A. T., Torbey, S., Hermann, K. M., Schnitzer, T. J., & Apkarian, A. V. (2013). Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain, 136(9), 2751-2768.
doi: 10.1093/brain/awt211 URL |
| [36] |
Huckins, J. F., Adeyemo, B., Miezin, F. M., Power, J. D., Gordon, E. M., Laumann, T. O., ... Kelley, W. M. (2019). Reward-related regions form a preferentially coupled system at rest. Human Brain Mapping, 40(2), 361-376.
doi: 10.1002/hbm.24377 pmid: 30251766 |
| [37] |
Jin, C., Yuan, K., Zhao, L., Zhao, L., Yu, D., von Deneen, K. M., ... Tian, J. (2013). Structural and functional abnormalities in migraine patients without aura. NMR in Biomedicine, 26(1), 58-64.
doi: 10.1002/nbm.2819 pmid: 22674568 |
| [38] |
Kamping, S., Bomba, I. C., Kanske, P., Diesch, E., & Flor, H. (2013). Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain, 154(9), 1846-1855.
doi: 10.1016/j.pain.2013.06.003 pmid: 23752177 |
| [39] |
Kehlet, H. (2018). Postoperative pain, analgesia, and recovery— Bedfellows that cannot be ignored. Pain, 159(1), S11-S16.
doi: 10.1097/j.pain.0000000000001243 URL |
| [40] |
Kim, D. J., Jassar, H., Lim, M., Nascimento, T. D., & DaSilva, A. F. (2021). Dopaminergic regulation of reward system connectivity underpins pain and emotional suffering in migraine. Journal of Pain Research, 14, 631-643.
doi: 10.2147/JPR.S296540 pmid: 33727857 |
| [41] |
Kim, M., Mawla, I., Albrecht, D. S., Admon, R., Torrado-Carvajal, A., Bergan, C., ... Loggia, M. L. (2020). Striatal hypofunction as a neural correlate of mood alterations in chronic pain patients. NeuroImage, 211, 116656.
doi: 10.1016/j.neuroimage.2020.116656 URL |
| [42] | Knutson, B., Fong, G. W., Adams, C. M., Varner, J. L., & Hommer, D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuro Report, 12(17), 3683-3687. |
| [43] |
Kocsel, N., Galambos, A., Szabó, E., Édes, A. E., Magyar, M., Zsombók, T., ... Juhász, G. (2019). Altered neural activity to monetary reward/loss processing in episodic migraine. Scientific Reports, 9(1), 5420.
doi: 10.1038/s41598-019-41867-x pmid: 30931979 |
| [44] |
Kurnianingsih, Y. A., & Mullette-Gillman, O. A. (2016). Neural mechanisms of the transformation from objective value to subjective utility: Converting from count to worth. Frontiers in Neuroscience, 10, 507.
pmid: 27881949 |
| [45] | Liu, X., Wang, N., Gu, L., Guo, J., Wang, J., & Luo, F. (2019). Reward processing under chronic pain from the perspective of “Liking” and “Wanting”: A Narrative Review. Pain Research and Management, 2019, 6760121. |
| [46] |
Liu, Y.-T., Shao, Y.-W., Yen, C.-T., & Shaw, F.-Z. (2014). Acid-induced hyperalgesia and anxio-depressive comorbidity in rats. Physiology & Behavior, 131, 105-110.
doi: 10.1016/j.physbeh.2014.03.030 URL |
| [47] | Loggia, M. L., Berna, C., Kim, J., Cahalan, C. M., Gollub, R. L., Wasan, A. D., ... Napadow, V. (2014). Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis & Rheumatology, 66(1), 203-212. |
| [48] |
Lonsdorf, T. B., & Richter, J. (2017). Challenges of fear conditioning research in the age of RDoC. Zeitschrift Für Psychologie, 225(3), 189-199.
doi: 10.1027/2151-2604/a000303 URL |
| [49] |
Low, L. A., & Fitzgerald, M. (2012). Acute pain and a motivational pathway in adult rats: Influence of early life pain experience. PLoS ONE, 7(3), e34316.
doi: 10.1371/journal.pone.0034316 URL |
| [50] |
Marbach, J. J., Richlin, D. M., & Lipton, J. A. (1983). Illness behavior, Depression and anhedonia in myofascial face and back pain patients. Psychotherapy and Psychosomatics, 39(1), 47-54.
doi: 10.1159/000287720 pmid: 6220421 |
| [51] |
Martucci, K. T., Borg, N., MacNiven, K. H., Knutson, B., & Mackey, S. C. (2018). Altered prefrontal correlates of monetary anticipation and outcome in chronic pain. Pain, 159(8), 1494-1507.
doi: 10.1097/j.pain.0000000000001232 pmid: 29790868 |
| [52] |
Martucci, K. T., MacNiven, K. H., Borg, N., Knutson, B., & Mackey, S. C. (2019). Apparent effects of opioid use on neural responses to reward in chronic pain. Scientific Reports, 9(1), 9633.
doi: 10.1038/s41598-019-45961-y pmid: 31270360 |
| [53] |
McFarland, B. R., Shankman, S. A., Tenke, C. E., Bruder, G. E., & Klein, D. N. (2006). Behavioral activation system deficits predict the six-month course of depression. Journal of Affective Disorders, 91(2-3), 229-234.
doi: 10.1016/j.jad.2006.01.012 pmid: 16487598 |
| [54] |
Mitsi, V., & Zachariou, V. (2016). Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience, 338, 81-92.
doi: S0306-4522(16)30166-X pmid: 27189881 |
| [55] |
Murray, C. J. L., & Lopez, A. D. (2013). Measuring the global burden of disease. New England Journal of Medicine, 369(5), 448-457.
doi: 10.1056/NEJMra1201534 URL |
| [56] |
Navratilova, E., Morimura, K., Xie, J. Y., Atcherley, C. W., Ossipov, M. H., & Porreca, F. (2016). Positive emotions and brain reward circuits in chronic pain. Journal of Comparative Neurology, 524(8), 1646-1652.
doi: 10.1002/cne.23968 pmid: 26788716 |
| [57] |
Nees, F., Becker, S., Millenet, S., Banaschewski, T., Poustka, L., Bokde, A., ··· IMAGEN consortium. (2017). Brain substrates of reward processing and the μ-opioid receptor: A pathway into pain? Pain, 158(2), 212-219.
doi: 10.1097/j.pain.0000000000000720 pmid: 28092323 |
| [58] |
Nees, F., Usai, K., Löffler, M., & Flor, H. (2019). The evaluation and brain representation of pleasant touch in chronic and subacute back pain. Neurobiology of Pain, 5, 100025.
doi: 10.1016/j.ynpai.2018.10.002 URL |
| [59] |
Nijs, J., Mairesse, O., Neu, D., Leysen, L., Danneels, L., Cagnie, B., ... Goubert, D. (2018). Sleep disturbances in chronic pain: Neurobiology, assessment, and treatment in physical therapist practice. Physical Therapy, 98(5), 325-335.
doi: 10.1093/ptj/pzy020 pmid: 29425327 |
| [60] |
Nusslock, R., & Alloy, L. B. (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3-16.
doi: S0165-0327(16)31001-1 pmid: 28237133 |
| [61] |
Okun, A., McKinzie, D. L., Witkin, J. M., Remeniuk, B., Husein, O., Gleason, S. D., ... Porreca, F. (2016). Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain, 157(12), 2731-2738.
doi: 10.1097/j.pain.0000000000000695 pmid: 27548047 |
| [62] |
Peciña, S., & Berridge, K. C. (2005). Hedonic hot spot in Nucleus Accumbens Shell: Where do mu-Opioids cause increased hedonic impact of sweetness? Journal of Neuroscience, 25(50), 11777-11786.
doi: 10.1523/JNEUROSCI.2329-05.2005 pmid: 16354936 |
| [63] |
Peciña, S., Cagniard, B., Berridge, K. C., Aldridge, J. W., & Zhuang, X. (2003). Hyperdopaminergic mutant mice have higher “Wanting” but not “Liking” for sweet rewards. The Journal of Neuroscience, 23(28), 9395-9402.
doi: 10.1523/JNEUROSCI.23-28-09395.2003 URL |
| [64] |
Porreca, F., & Navratilova, E. (2017). Reward, motivation, and emotion of pain and its relief. Pain, 158(1), S43-S49.
doi: 10.1097/j.pain.0000000000000798 URL |
| [65] |
Rizvi, S. J., Gandhi, W., & Salomons, T. (2021). Reward processing as a common diathesis for chronic pain and depression. Neuroscience & Biobehavioral Reviews, 127, 749-760.
doi: 10.1016/j.neubiorev.2021.04.033 URL |
| [66] |
Roughan, W. H., Campos, A. I., García-Marín, L. M., Cuéllar-Partida, G., Lupton, M. K., Hickie, I. B., ... Rentería, M. E. (2021). Comorbid chronic pain and depression: Shared risk factors and differential antidepressant effectiveness. Frontiers in Psychiatry, 12, 643609.
doi: 10.3389/fpsyt.2021.643609 URL |
| [67] |
Salcido, C. A., Harris Bozer, A. L., McNabb, C. T., & Fuchs, P. N. (2018). Assessing the aversive nature of pain with an operant approach/avoidance paradigm. Physiology & Behavior, 189, 59-63.
doi: 10.1016/j.physbeh.2018.02.053 URL |
| [68] |
Schwartz, N., Miller, C., & Fields, H. L. (2017). Cortico- Accumbens regulation of approach-avoidance behavior is modified by experience and chronic pain. Cell Reports, 19(8), 1522-1531.
doi: S2211-1247(17)30602-2 pmid: 28538173 |
| [69] |
Scott, D. J., Heitzeg, M. M., Koeppe, R. A., Stohler, C. S., & Zubieta, J.-K. (2006). Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. Journal of Neuroscience, 26(42), 10789-10795.
doi: 10.1523/JNEUROSCI.2577-06.2006 pmid: 17050717 |
| [70] | Sheng, J., Liu, S., Wang, Y., Cui, R., & Zhang, X. (2017). The link between depression and chronic pain: Neural mechanisms in the brain. Neural Plasticity, 2017, 9724371. |
| [71] |
Small, D. M., & Apkarian, V. A. (2006). Increased taste intensity perception exhibited by patients with chronic back pain. Pain, 120(1-2), 124-130.
doi: 10.1016/j.pain.2005.10.021 pmid: 16360267 |
| [72] |
Taylor, A. M. W., Becker, S., Schweinhardt, P., & Cahill, C. (2016). Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain, 157(6), 1194-1198.
doi: 10.1097/j.pain.0000000000000494 pmid: 26797678 |
| [73] |
Thompson, S. J., Pitcher, M. H., Stone, L. S., Tarum, F., Niu, G., Chen, X., ... Bushnell, M. C. (2018). Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain, 159(9), 1856-1866.
doi: 10.1097/j.pain.0000000000001282 pmid: 29794614 |
| [74] |
Wang, C., Bao, C., Gao, J., Gu, Y., & Dong, X. (2020). Pain modulates neural responses to reward in the medial prefrontal cortex. Human Brain Mapping, 41(5), 1372-1381.
doi: 10.1002/hbm.24882 pmid: 31785068 |
| [75] |
Wang, C., Gao, J., Ma, Y., Zhu, C., & Dong, X.-W. (2018). Physical pain increases interpersonal trust in females. European Journal of Pain, 22(1), 150-160.
doi: 10.1002/ejp.1111 pmid: 28913979 |
| [76] | Watanabe, M., & Narita, M. (2018). Brain reward circuit and pain. In B.-C. Shyu & M. Tominaga (Eds.), Advances in pain research: Mechanisms and modulation of chronic pain (Vol. 1099, pp. 201-210). Springer Singapore. |
| [77] |
Wood, P. B., Schweinhardt, P., Jaeger, E., Dagher, A., Hakyemez, H., Rabiner, E. A., Bushnell, M. C., & Chizh, B. A. (2007). Fibromyalgia patients show an abnormal dopamine response to pain. European Journal of Neuroscience, 25(12), 3576-3582.
doi: 10.1111/j.1460-9568.2007.05623.x pmid: 17610577 |
| [78] |
Yang, X., Liu, X., Zeng, Y., Wu, R., Zhao, W., Xin, F., ... Becker, B. (2021). Secondary rewards acquire enhanced incentive motivation via increasing anticipatory activity of the lateral orbitofrontal cortex. Brain Structure and Function, 226(7), 2339-2355.
doi: 10.1007/s00429-021-02333-5 pmid: 34254166 |
| [79] |
Yarkoni, T., Poldrack, R. A., Nichols, T. E., van Essen, D. C., & Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665-670.
doi: 10.1038/nmeth.1635 pmid: 21706013 |
| [80] |
Zald, D. H., & Treadway, M. T. (2017). Reward processing, neuroeconomics, and psychopathology. Annual Review of Clinical Psychology, 13(1), 471-495.
doi: 10.1146/annurev-clinpsy-032816-044957 URL |
| [81] |
Zubieta, J.-K., Smith, Y. R., Bueller, J. A., Kilbourn, M. R., Jewett, D. M., ... Stohler, C. S. (2001). Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science, 293(5528), 311-315.
doi: 10.1126/science.1060952 pmid: 11452128 |
| [1] | 丁颖, 汪紫滢, 李卫东. 抑郁症疼痛加工的行为特点及神经机制[J]. 心理科学进展, 2024, 32(8): 1315-1327. |
| [2] | 周冰涛, 刘宇平, 赵辉, 杨波. 攻击行为的愉悦效应[J]. 心理科学进展, 2023, 31(9): 1714-1727. |
| [3] | 寇娟, 杨梦圆, 魏子杰, 雷怡. 自闭症谱系障碍社交动机理论:机制及干预探索[J]. 心理科学进展, 2023, 31(1): 20-32. |
| [4] | 王松雪, 程思, 蒋挺, 刘勋, 张明霞. 外在奖赏对陈述性记忆的影响[J]. 心理科学进展, 2023, 31(1): 78-86. |
| [5] | 刘博, 程香娟, 岳衡, 包呼格吉乐图. 抑制功能在疼痛中的作用[J]. 心理科学进展, 2022, 30(6): 1253-1261. |
| [6] | 徐慧, 王滔. 自闭症谱系障碍个体的社会动机缺陷[J]. 心理科学进展, 2022, 30(5): 1050-1061. |
| [7] | 顾丽佳, 宫文潇, 张静, 陈巍, 郭建友. 身体拥有感错觉对疼痛的影响及其作用机制[J]. 心理科学进展, 2022, 30(11): 2518-2528. |
| [8] | 严万森, 刘苏姣, 张冉冉, 徐鹏. 强迫性特征在药物成瘾行为中的易感性及其前额叶-反奖赏系统神经基础[J]. 心理科学进展, 2021, 29(8): 1345-1357. |
| [9] | 秦浩方, 黄蓉, 贾世伟. 反馈相关负波:一种抑郁症的生物标记物[J]. 心理科学进展, 2021, 29(3): 404-413. |
| [10] | 王磊, 贺荟中, 毕小彬, 周丽, 范晓壮. 社会动机理论视角下自闭症谱系障碍者的社交缺陷[J]. 心理科学进展, 2021, 29(12): 2209-2223. |
| [11] | 周璨, 周临舒, 蒋存梅. 音乐愉悦体验的神经机制[J]. 心理科学进展, 2021, 29(1): 123-130. |
| [12] | 陈乐乐, 黄蓉, 贾世伟. 反馈相关负波与成瘾[J]. 心理科学进展, 2020, 28(6): 959-968. |
| [13] | 刘昕鹤, 王宁, 王锦琰, 罗非. 疼痛背景下时距知觉的变化[J]. 心理科学进展, 2020, 28(5): 766-777. |
| [14] | 秦楠, 张明霞. 基于奖赏学习的注意对对比度知觉的影响[J]. 心理科学进展, 2019, 27(suppl.): 25-25. |
| [15] | 黄红, 孟明. 人脑奖赏系统对观看短视频认知评价的影响[J]. 心理科学进展, 2019, 27(suppl.): 152-152. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||