

CN 11-1911/B
主办:中国心理学会
中国科学院心理研究所
出版:科学出版社


心理学报 ›› 2022, Vol. 54 ›› Issue (6): 604-612.doi: 10.3724/SP.J.1041.2022.00604 cstr: 32110.14.2022.00604
收稿日期:2021-08-17
发布日期:2022-04-26
出版日期:2022-06-25
基金资助:
ZHOU Ping1, XIAO Hua1, LI Yonghui2,3, DONG Xinwen2( )
)
Received:2021-08-17
Online:2022-04-26
Published:2022-06-25
摘要:
剧烈的应激刺激会引起持续的高唤醒状态, 是多种应激障碍的核心症状, 并推进其他症状的发生发展。本研究关注5-羟色胺在应激诱发高唤醒的发生、发展中的作用, 通过测量听觉惊吓反射水平反映高唤醒状态, 考察色氨酸羟化酶-2基因缺陷小鼠在天敌或电击应激前后高唤醒的变化。研究发现, 雄性基因缺陷小鼠在应激后出现持续一周以上的高唤醒表现, 而野生型小鼠高唤醒状态很快恢复。结果提示, 基因缺陷引起的5-羟色胺降低可能是强应激诱发的持续高唤醒的易感因素。
中图分类号:
周萍, 肖华, 李勇辉, 董昕文. (2022). 5-羟色胺基因缺陷增强急性应激后高唤醒状态. 心理学报, 54(6), 604-612.
ZHOU Ping, XIAO Hua, LI Yonghui, DONG Xinwen. (2022). Sustained hyperarousal induced by acute stress in tryptophan-hydroxylase-2 genetic deficient male mice. Acta Psychologica Sinica, 54(6), 604-612.

图1 性别、基因型对小鼠听觉惊吓反射幅值的影响注:误差线为标准误(SEM)。各组例数:雄性野生型, n = 26; 雄性Tph2 +/-, n = 37; 雌性野生型, n = 31; 雌性Tph2 +/-, n = 29。雄性小鼠惊吓反射基线值显著高于雌性小鼠, *** p < 0.001, 同一性别不同基因型小鼠之间惊吓反射基线值无差别。
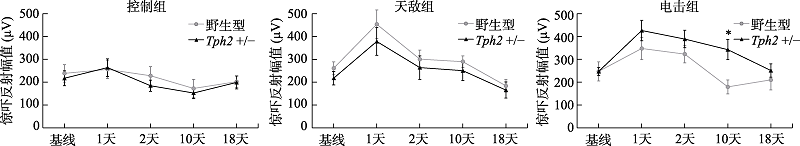
图2 雄性小鼠在应激前后听觉惊吓反射幅值变化注:误差线为标准误(SEM)。各组例数:野生型控制组, n = 8, 天敌组, n = 9, 电击组, n = 9; Tph2 +/-控制组, n = 13, 天敌组, n = 10只, 电击组, n = 14。图中*标记指示电击应激后10天, 不同基因型的雄性小鼠间的惊吓反射幅度差异, * p < 0.05。
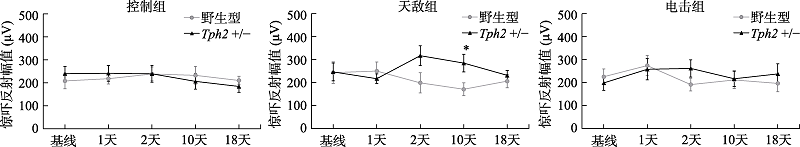
图3 雌性小鼠在应激前后听觉惊吓反射幅值变化注:误差线为标准误(SEM)。各组例数:野生型控制组, n = 12, 天敌组, n = 8, 电击组, n = 11; Tph2 +/-雌鼠控制组, n = 12, 天敌组, n = 6, 电击组, n = 11。图中*标记指示天敌暴露应激后10天, 不同基因型的雌性小鼠间的惊吓反射幅度差异, * p < 0.05。
| [1] |
Abela, A. R., Browne, C. J., Sargin, D., Prevot, T. D., Ji, X. D., Li, Z., Lambe, E. K., & Fletcher, P. J. (2020). Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology, 168, 107985. https://doi.org/10.1016/j.neuropharm.2020.107985
doi: 10.1016/j.neuropharm.2020.107985 URL |
| [2] | Agarwal, T. M., Muneer, M., Asim, M., Awad, M., Afzal, Y., Al-Thani, H., Alhassan, A., Mollazehi, M., & El-Menyar, A. (2020). Psychological trauma in different mechanisms of traumatic injury: A hospital-based cross-sectional study. PLoS ONE, 15(11), e0242849. https://doi.org/10.1371/journal.pone.0242849 |
| [3] | Akiki, T. J., & Abdallah, C. G. (2018). Are There Effective Psychopharmacologic Treatments for PTSD? The Journal of Clinical Psychiatry, 80(3), 18ac12473. https://doi.org/10.4088/JCP.18ac12473 |
| [4] | American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596 |
| [5] |
Auth, C. S., Weidner, M. T., Popp, S., Strekalova, T., Schmitt-Böhrer, A. G., van den Hove, D. L., Lesch, K.-P., & Waider, J. (2018). Differential anxiety-related behaviours and brain activation in Tph2-deficient female mice exposed to adverse early environment. European Neuropsychopharmacology, 28(11), 1270-1283. https://doi.org/10.1016/j.euroneuro.2018.07.103
doi: 10.1016/j.euroneuro.2018.07.103 URL |
| [6] |
Bernabe, C. S., Caliman, I. F., Truitt, W. A., Molosh, A. I., Lowry, C. A., Hay-Schmidt, A., Shekhar, A., & Johnson, P. L. (2020). Using loss- and gain-of-function approaches to target amygdala-projecting serotonergic neurons in the dorsal raphe nucleus that enhance anxiety-related and conditioned fear behaviors. Journal of Psychopharmacology, 34(4), 400-411. https://doi.org/10.1177/0269881119900981
doi: 10.1177/0269881119900981 URL |
| [7] |
Boal, A. L., Vaughan, C. A., Sims, C. S., & Miles, J. N. V. (2017). Measurement invariance across administration mode: Examining the Posttraumatic Stress Disorder (PTSD) Checklist. Psychological Assessment, 29(1), 76-86. https://doi.org/10.1037/pas0000301
doi: 10.1037/pas0000301 URL |
| [8] | Brivio, P., Sbrini, G., Peeva, P., Todiras, M., Bader, M., Alenina, N., & Calabrese, F. (2018). TPH2 deficiency influences neuroplastic mechanisms and alters the response to an acute stress in a sex specific manner. Frontiers in Molecular Neuroscience, 11, 389. https://doi.org/10.3389/fnmol.2018.00389 |
| [9] |
Bryant, R. A., Creamer, M., O’Donnell, M., Forbes, D., McFarlane, A. C., Silove, D., & Hadzi-Pavlovic, D. (2017). Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: A network analysis. JAMA Psychiatry, 74(2), 135-142. https://doi.org/10.1001/jamapsychiatry.2016.3470
doi: 10.1001/jamapsychiatry.2016.3470 URL |
| [10] |
Bryant, R. A., Creamer, M., O’Donnell, M., Silove, D., & McFarlane, A. C. (2008). A multisite study of initial respiration rate and heart rate as predictors of posttraumatic stress disorder. The Journal of Clinical Psychiatry, 69(11), 1694-1701. https://doi.org/10.4088/JCP.v69n1104
doi: 10.4088/JCP.v69n1104 URL |
| [11] |
Cao, C., Wang, L., Wang, R., Qing, Y., & Zhang, J. (2014). TPH2 genotype is associated with PTSD’s avoidance symptoms in Chinese female earthquake survivors. Psychiatric Genetics, 24(6), 257-261. https://doi.org/10.1097/YPG.0000000000000048
doi: 10.1097/YPG.0000000000000048 URL |
| [12] |
Cohen, H., Kaplan, Z., Koresh, O., Matar, M. A., Geva, A. B., & Zohar, J. (2011). Early post-stressor intervention with propranolol is ineffective in preventing posttraumatic stress responses in an animal model for PTSD. European Neuropsychopharmacology, 21(3), 230-240. https://doi.org/10.1016/j.euroneuro.2010.11.011
doi: 10.1016/j.euroneuro.2010.11.011 URL |
| [13] | Cohen, H., & Zohar, J. (2004). An animal model of posttraumatic stress disorder:The use of cut-off behavioral criteria. Annals of the New York Academy of Sciences, 1032(1), 167-178. https://doi.org/10.1196/annals.1314.014 |
| [14] |
Coronas, R., Gallardo, O., Moreno, M. J., Suárez, D., García-Parés, G., & Menchón, J. M. (2011). Heart rate measured in the acute aftermath of trauma can predict post-traumatic stress disorder: A prospective study in motor vehicle accident survivors. European Psychiatry, 26(8), 508-512. https://doi.org/10.1016/j.eurpsy.2010.06.006
doi: 10.1016/j.eurpsy.2010.06.006 URL pmid: 20813504 |
| [15] |
da Silva Soares, R., Falconi-Sobrinho, L. L., Almada, R. C., & Coimbra, N. C. (2019). Dorsal raphe nucleus 5-Hydroxytryptamine 2A receptors are critical for the organisation of panic attack-like defensive behaviour and unconditioned fear-induced antinociception elicited by the chemical stimulation of superior colliculus neurons. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 29(7), 858-870. https://doi.org/10.1016/j.euroneuro.2019.05.007
doi: 10.1016/j.euroneuro.2019.05.007 URL |
| [16] | Dong, X., & Li, Y. (2014). Peritraumatic startle response predicts the vulnerability to develop PTSD-like behaviors in rats: A model for peritraumatic dissociation. Frontiers in Behavioral Neuroscience, 8, 14. https://doi.org/10.3389/fnbeh.2014.00014 |
| [17] |
Gelkopf, M., Lapid Pickman, L., Carlson, E. B., & Greene, T. (2019). The dynamic relations among peritraumatic posttraumatic stress symptoms: An experience sampling study during wartime. Journal of Traumatic Stress, 32(1), 119-129. https://doi.org/10.1002/jts.22374
doi: 10.1002/jts.22374 URL pmid: 30720893 |
| [18] |
Gonzales, M., Garrett, C., Chapman, C. D., & Dess, N. K. (2008). Stress-induced attenuation of acoustic startle in low-saccharin-consuming rats. Biological Psychology, 79(2), 193-199. https://doi.org/10.1016/j.biopsycho.2008.04.011
doi: 10.1016/j.biopsycho.2008.04.011 URL pmid: 18538914 |
| [19] |
Greene, T., Gelkopf, M., Fried, E. I., Robinaugh, D. J., & Lapid Pickman, L. (2020). Dynamic network analysis of negative emotions and DSM-5 posttraumatic stress disorder symptom clusters during conflict. Journal of Traumatic Stress, 33(1), 72-83. https://doi.org/10.1002/jts.22433
doi: 10.1002/jts.22433 URL pmid: 31433530 |
| [20] |
Gross, C. T., & Canteras, N. S. (2012). The many paths to fear. Nature Reviews Neuroscience, 13(9), 651-658. https://doi.org/10.1038/nrn3301
doi: 10.1038/nrn3301 URL |
| [21] |
Gutknecht, L., Araragi, N., Merker, S., Waider, J., Sommerlandt, F. M. J., Mlinar, B., Baccini, G., Mayer, U., Proft, F., Hamon, M., Schmitt, A. G., Corradetti, R., Lanfumey, L., & Lesch, K.-P. (2012). Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS ONE, 7(8), e43157. https://doi.org/10.1371/journal.pone.0043157
doi: 10.1371/journal.pone.0043157 URL |
| [22] |
Hubbard, C. S., Ornitz, E., Gaspar, J. X., Smith, S., Amin, J., Labus, J. S., Kilpatrick, L. A., Rhudy, J. L., Mayer, E. A., & Naliboff, B. D. (2011). Modulation of nociceptive and acoustic startle responses to an unpredictable threat in men and women. Pain, 152(7), 1632-1640. https://doi.org/10.1016/j.pain.2011.03.001
doi: 10.1016/j.pain.2011.03.001 URL |
| [23] |
Jacobsen, J. P. R., Siesser, W. B., Sachs, B. D., Peterson, S., Cools, M. J., Setola, V., Folgering, J. H. A., Flik, G., & Caron, M. G. (2012). Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Molecular Psychiatry, 17(7), 694-704. https://doi.org/10.1038/mp.2011.50
doi: 10.1038/mp.2011.50 URL pmid: 21537332 |
| [24] | Kaehler, S. T., Singewald, N., Sinner, C., Thurnher, C., & Philippu, A. (2000). Conditioned fear and inescapable shock modify the release of serotonin in the locus coeruleus. Brain Research, 859(2), 249-254. https://doi.org/10.1016/s0006-8993(00)01967-3 |
| [25] |
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., & Nelson, C. B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048-1060. https://doi.org/10.1001/archpsyc.1995.03950240066012
doi: 10.1001/archpsyc.1995.03950240066012 URL pmid: 7492257 |
| [26] |
Kilpatrick, D. G., Resnick, H. S., Milanak, M. E., Miller, M. W., Keyes, K. M., & Friedman, M. J. (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26(5), 537-547. https://doi.org/10.1002/jts.21848
doi: 10.1002/jts.21848 URL pmid: 24151000 |
| [27] |
Kobayashi, I., & Mellman, T. A. (2012). Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behavioral Sleep Medicine, 10(3), 180-190. https://doi.org/10.1080/15402002.2011.654296
doi: 10.1080/15402002.2011.654296 URL pmid: 22742436 |
| [28] | Kolter, J. F., Hildenbrand, M. F., Popp, S., Nauroth, S., Bankmann, J., Rother, L., Waider, J., Deckert, J., Asan, E., Jakob, P. M., Lesch, K.-P., & Schmitt-Böhrer, A. (2021). Serotonin transporter genotype modulates resting state and predator stress-induced amygdala perfusion in mice in a sex-dependent manner. PloS One, 16(2), e0247311. https://doi.org/10.1371/journal.pone.0247311 |
| [29] |
Koresh, O., Kaplan, Z., Zohar, J., Matar, M. A., Geva, A. B., & Cohen, H. (2016). Distinctive cardiac autonomic dysfunction following stress exposure in both sexes in an animal model of PTSD. Behavioural Brain Research, 308, 128-142. https://doi.org/10.1016/j.bbr.2016.04.024
doi: 10.1016/j.bbr.2016.04.024 URL pmid: 27105958 |
| [30] |
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1990). Emotion, attention, and the startle reflex. Psychological Review, 97(3), 377-395.
pmid: 2200076 |
| [31] |
Lieb, M. W., Weidner, M., Arnold, M. R., Loupy, K. M., Nguyen, K. T., Hassell, J. E., Schnabel, K. S., Kern, R., Day, H. E. W., Lesch, K.-P., Waider, J., & Lowry, C. A. (2019). Effects of maternal separation on serotonergic systems in the dorsal and median raphe nuclei of adult male Tph2-deficient mice. Behavioural Brain Research, 373, 112086. https://doi.org/10.1016/j.bbr.2019.112086
doi: 10.1016/j.bbr.2019.112086 URL |
| [32] |
Linthorst, A. C. E., & Reul, J. M. (2008). Stress and the brain: Solving the puzzle using microdialysis. Pharmacology Biochemistry and Behavior, 90(2), 163-173. https://doi.org/10.1016/j.pbb.2007.09.019
URL pmid: 18028991 |
| [33] |
Liu, Y., Jiang, Y., Si, Y., Kim, J.-Y., Chen, Z.-F., & Rao, Y. (2011). Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature, 472(7341), 95-99. https://doi.org/10.1038/nature09822
doi: 10.1038/nature09822 URL |
| [34] | McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. https://doi.org/10.1177/2470547017692328 |
| [35] |
McQuade, R., & Sharp, T. (2002). Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. Journal of Neurochemistry, 69(2), 791-796. https://doi.org/10.1046/j.1471-4159.1997.
doi: 10.1046/j.1471-4159.1997.69020791.x URL |
| [36] |
Monti, J. M. (2011). Serotonin control of sleep-wake behavior. Sleep Medicine Reviews, 15(4), 269-281. https://doi.org/10.1016/j.smrv.2010.11.003
doi: 10.1016/j.smrv.2010.11.003 URL |
| [37] |
Mosienko, V., Matthes, S., Hirth, N., Beis, D., Flinders, M., Bader, M., Hansson, A. C., & Alenina, N. (2014). Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology, 85, 73-80. https://doi.org/10.1016/j.neuropharm.2014.05.015
doi: 10.1016/j.neuropharm.2014.05.015 URL |
| [38] |
Russo, A. M., Lawther, A. J., Prior, B. M., Isbel, L., Somers, W. G., Lesku, J. A., Richdale, A. L., Dissanayake, C., Kent, S., Lowry, C. A., & Hale, M. W. (2019). Social approach, anxiety, and altered tryptophan hydroxylase 2 activity in juvenile BALB/c and C57BL/6J mice. Behavioural Brain Research, 359, 918-926. https://doi.org/10.1016/j.bbr.2018.06.019
doi: 10.1016/j.bbr.2018.06.019 URL |
| [39] |
Sachs, B. D., Ni, J. R., & Caron, M. G. (2015). Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proceedings of the National Academy of Sciences, 112(8), 2557-2562. https://doi.org/10.1073/pnas.1416866112
doi: 10.1073/pnas.1416866112 URL |
| [40] |
Savelieva, K. V., Zhao, S., Pogorelov, V. M., Rajan, I., Yang, Q., Cullinan, E., & Lanthorn, T. H. (2008). Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE, 3(10), e3301. https://doi.org/10.1371/journal.pone.0003301
doi: 10.1371/journal.pone.0003301 URL |
| [41] |
Sengupta, A., & Holmes, A. (2019). A discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron, 103(3), 489-505.e7. https://doi.org/10.1016/j.neuron.2019.05.029
doi: S0896-6273(19)30482-9 URL pmid: 31204082 |
| [42] |
Shaikh al arab, A., Guédon-Moreau, L., Ducrocq, F., Molenda, S., Duhem, S., Salleron, J., Chaudieu, I., Bert, D., Libersa, C., & Vaiva, G. (2012). Temporal analysis of heart rate variability as a predictor of post traumatic stress disorder in road traffic accidents survivors. Journal of Psychiatric Research, 46(6), 790-796. https://doi.org/10.1016/j.jpsychires.2012.02.006
doi: 10.1016/j.jpsychires.2012.02.006 URL pmid: 22425487 |
| [43] |
Sherin, J. E., & Nemeroff, C. B. (2011). Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues in Clinical Neuroscience, 13(3), 263-278. https://doi.org/10.31887/DCNS.2011.13.2/jsherin
doi: 10.31887/DCNS.2011.13.2/jsherin URL |
| [44] | Sijbrandij, M., Kleiboer, A., Bisson, J. I., Barbui, C., & Cuijpers, P. (2015). Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: A systematic review and meta-analysis. The Lancet. Psychiatry, 2(5), 413-421. https://doi.org/10.1016/S2215-0366(14)00121-7 |
| [45] |
Smith, K. L., Kassem, M. S., Clarke, D. J., Kuligowski, M. P., Bedoya-Pérez, M. A., Todd, S. M., Lagopoulos, J., Bennett, M. R., & Arnold, J. C. (2019). Microglial cell hyper- ramification and neuronal dendritic spine loss in the hippocampus and medial prefrontal cortex in a mouse model of PTSD. Brain, Behavior, and Immunity, 80, 889-899. https://doi.org/10.1016/j.bbi.2019.05.042
doi: 10.1016/j.bbi.2019.05.042 URL |
| [46] |
Stam, R. (2007). PTSD and stress sensitisation: A tale of brain and body Part 2: Animal models. Neuroscience & Biobehavioral Reviews, 31(4), 558-584. https://doi.org/10.1016/j.neubiorev.2007.01.001
doi: 10.1016/j.neubiorev.2007.01.001 URL |
| [47] | Strekalova, T., Svirin, E., Waider, J., Gorlova, A., Cespuglio, R., Kalueff, A., Pomytkin, I., Schmitt-Boehrer, A. G., Lesch, K.-P., & Anthony, D. C. (2021). Altered behaviour, dopamine and norepinephrine regulation in stressed mice heterozygous in TPH2 gene. Progress in Neuro- Psychopharmacology and Biological Psychiatry, 108, 110155. https://doi.org/10.1016/j.pnpbp.2020.110155 |
| [48] |
Tao, S., Chattun, M. R., Yan, R., Geng, J., Zhu, R., Shao, J., Lu, Q., & Yao, Z. (2018). TPH-2 gene polymorphism in major depressive disorder patients with early-wakening symptom. Frontiers in Neuroscience, 12, 827. https://doi. org/10.3389/fnins.2018.00827
doi: 10.3389/fnins.2018.00827 URL |
| [49] | Thakur, A., Choudhary, D., Kumar, B., & Chaudhary, A. (2021). A review on post-traumatic stress disorder (PTSD): “Symptoms, therapies and recent case studies.”. Current Molecular Pharmacology. https://doi.org/10.2174/1874467214666210525160944 |
| [50] |
Török, B., Sipos, E., Pivac, N., & Zelena, D. (2019). Modelling posttraumatic stress disorders in animals. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 90, 117-133. https://doi.org/10.1016/j.pnpbp.2018.11.013
doi: 10.1016/j.pnpbp.2018.11.013 URL |
| [51] |
Verona, E., & Kilmer, A. (2007). Stress exposure and affective modulation of aggressive behavior in men and women. Journal of Abnormal Psychology, 116(2), 410-421. https://doi.org/10.1037/0021-843X.116.2.410
doi: 10.1037/0021-843X.116.2.410 URL |
| [52] | Voulo, M. E., & Parsons, R. G. (2017). Response-specific sex difference in the retention of fear extinction. Learning & Memory, 24(6), 245-251. https://doi.org/10.1101/lm.045641.117 |
| [53] |
Voulo, M. E., & Parsons, R. G. (2019). Gonadal hormone fluctuations do not affect the expression or extinction of fear-potentiated startle in female rats. Behavioral Neuroscience, 133(5), 517-526. https://doi.org/10.1037/bne0000324
doi: 10.1037/bne0000324 URL |
| [54] | Walther, D. J., Peter, J.-U., Bashammakh, S., Hörtnagl, H., Voits, M., Fink, H., & Bader, M. (2003). Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science (New York, N.Y.), 299(5603), 76. https://doi.org/10.1126/science.1078197 |
| [55] |
Wankhar, W., Syiem, D., Pakyntein, C. L., Thabah, D., & Sunn, S. E. (2020). Effect of 5-HT2C receptor agonist and antagonist on chronic unpredictable stress (CUS) - Mediated anxiety and depression in adolescent Wistar albino rat: Implicating serotonin and mitochondrial ETC-I function in serotonergic neurotransmission. Behavioural Brain Research, 393, 112780. https://doi.org/10.1016/j.bbr.2020.112780
doi: 10.1016/j.bbr.2020.112780 URL |
| [56] |
Weidner, M. T., Lardenoije, R., Eijssen, L., Mogavero, F., de Groodt, L. P. M. T., Popp, S., Palme, R., Förstner, K. U., Strekalova, T., Steinbusch, H. W. M., Schmitt-Böhrer, A. G.., Glennon, J. C., Waider, J., van den Hove, D. L. A., & Lesch, K.-P.(2019). Identification of cholecystokinin by genome-wide profiling as potential mediator of serotonin- dependent behavioral effects of maternal separation in the amygdala. Frontiers in Neuroscience, 13, 460. https://doi.org/10.3389/fnins.2019.00460
doi: 10.3389/fnins.2019.00460 URL |
| [57] | Xu, Z., Reynolds, G. P., Yuan, Y., Shi, Y., Pu, M., & Zhang, Z. (2016). TPH-2 polymorphisms interact with early life stress to influence response to treatment with antidepressant drugs. International Journal of Neuropsychopharmacology, 19(11), pyw070. https://doi.org/10.1093/ijnp/pyw070 |
| [58] | Young, S. (2013). Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. Journal of Psychiatry & Neuroscience, 38(5), 294-305. https://doi.org/10.1503/jpn.120209 |
| [59] |
Zoladz, P. R., D’Alessio, P. A., Seeley, S. L., Kasler, C. D., Goodman, C. S., Mucher, K. E., Allison, A. S., Smith, I. F., Dodson, J. L., Stoops, T. S., & Rorabaugh, B. R. (2019). A predator-based psychosocial stress animal model of PTSD in females: Influence of estrous phase and ovarian hormones. Hormones and Behavior, 115, 104564. https://doi.org/10.1016/j.yhbeh.2019.104564
doi: 10.1016/j.yhbeh.2019.104564 URL |
| [1] | 李江娜;安书成;李珍. 应激性抑郁样行为发生中眶额叶5-HT1A受体对谷氨酸和γ-氨基丁酸的调节[J]. 心理学报, 2015, 47(10): 1269-1278. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||