

CN 11-1911/B


Acta Psychologica Sinica ›› 2024, Vol. 56 ›› Issue (10): 1351-1366.doi: 10.3724/SP.J.1041.2024.01351
• Reports of Empirical Studies • Previous Articles Next Articles
DU Xiayu1,2,3, LAI Lizu1,2,3, SHI Congrong1,2,3, GUO Zihan1,2,3, HAN Jing1,2,3, ZHANG Tao1,2,3, REN Zhihong1,2,3( )
)
Published:2024-10-25
Online:2024-07-10
Contact:
REN Zhihong
E-mail:ren@ccnu.edu.cn
DU Xiayu, LAI Lizu, SHI Congrong, GUO Zihan, HAN Jing, ZHANG Tao, REN Zhihong. (2024). Internet-based cognitive bias modification of interpretation in health anxiety: A randomized controlled trial. Acta Psychologica Sinica, 56(10), 1351-1366.
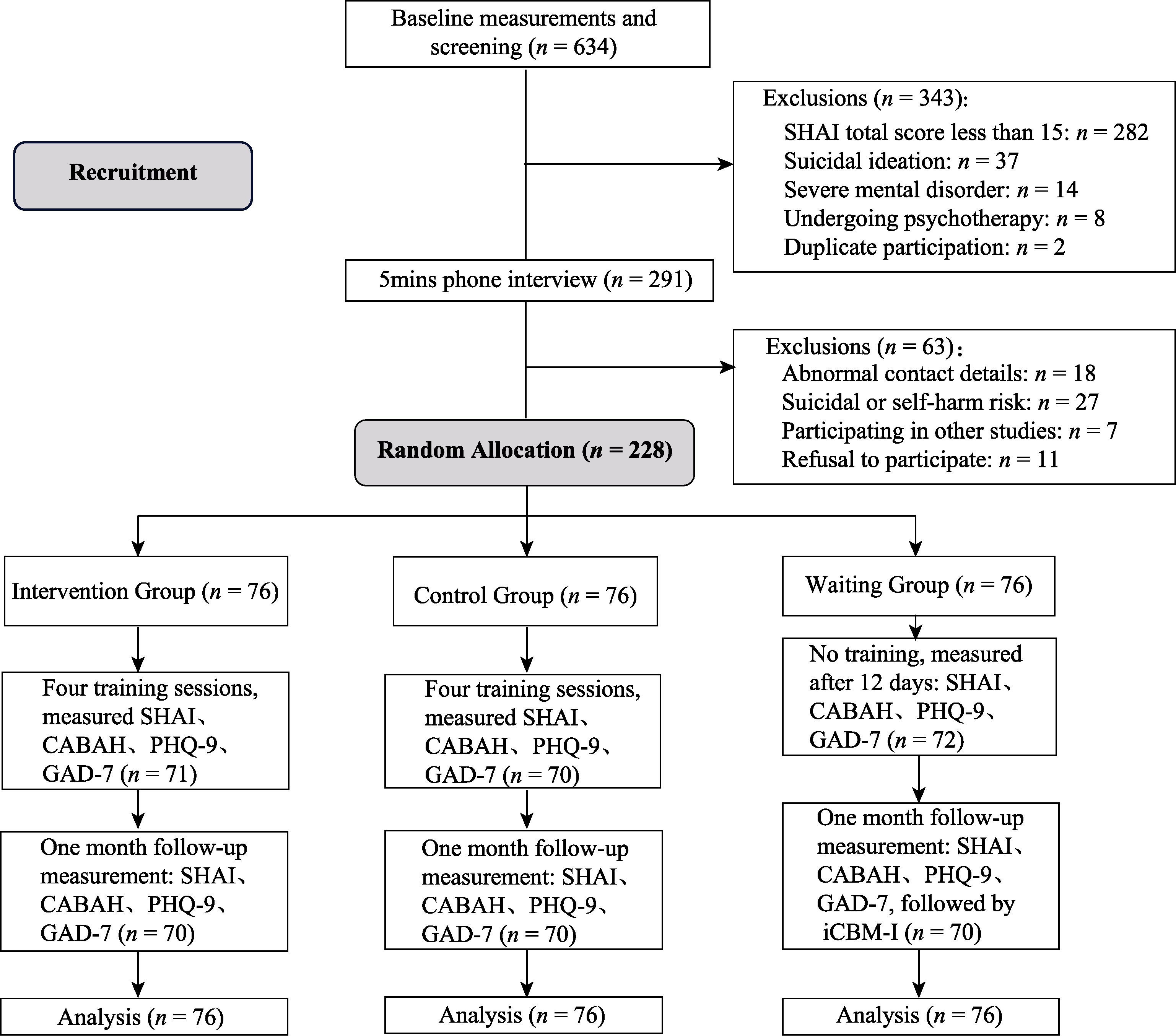
Figure 1. Flowchart of subject screening and intervention. Note. SHAI: Short Form Health Anxiety Inventory; CABAH: Cognitive Body and Health Inventory; PHQ-9: Patient Health Questionnaire; GAD-7: Generalized Anxiety Disorder Scale.
| outcome variable | Intervention group (n = 76) | Control group (n = 76) | Waiting group (n = 76) | Difference between groups | Intergroup effect sizes (Cohen's d, 95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F (2, 671) | Intervention group vs. waiting group | Control Group vs. Waiting Group | Intervention group vs. control group | ||
| SHAI | base line | 27.64 | 8.53 | 28.20 | 9.95 | 29.45 | 8.75 | 0.852 | ?0.21 (?0.53, 0.11) | ?0.14 (?0.46, 0.17) | ?0.07 (?0.38, 0.25) |
| post-test | 22.41 | 9.03 | 26.64 | 9.41 | 24.27 | 8.51 | 4.767** | ?0.21 (?0.53, 0.11) | 0.27(?0.05, 0.59) | ?0.50 (?0.82, ?0.18) | |
| follow up | 19.98 | 9.14 | 22.17 | 8.92 | 25.11 | 8.69 | 6.574** | ?0.59 (?0.91, ?0.26) | ?0.34 (?0.66, ?0.02) | ?0.26 (?0.58, 0.06) | |
| CABAH | base line | 36.54 | 6.07 | 36.79 | 6.40 | 35.00 | 6.31 | 2.343 | 0.28(?0.04, 0.60) | 0.33 (0.01, 0.65) | ?0.05 (?0.36, 0.27) |
| post-test | 29.22 | 6.74 | 32.22 | 6.47 | 33.77 | 5.60 | 13.835*** | ?0.83 (?1.16, ?0.50) | ?0.28 (?0.60, 0.04) | ?0.56 (?0.89, ?0.24) | |
| follow up | 29.87 | 7.30 | 32.48 | 6.20 | 34.05 | 5.97 | 11.471*** | ?0.76 (?1.09, ?0.44) | ?0.29 (?0.61, 0.03) | ?0.49 (?0.81, ?0.17) | |
| PHQ-9 | base line | 19.12 | 4.95 | 19.80 | 5.71 | 20.33 | 4.97 | 1.063 | -0.23 (?0.55, 0.08) | ?0.10 (?0.42, 0.22) | ?0.14 (?0.45, 0.18) |
| post-test | 15.79 | 4.12 | 17.29 | 5.46 | 20.59 | 4.74 | 17.244*** | ?0.94 (?1.27, ?0.60) | ?0.64 (?0.97, ?0.32) | ?0.30 (?0.62, 0.02) | |
| follow up | 16.72 | 4.35 | 18.26 | 5.75 | 20.64 | 4.10 | 11.212*** | ?0.77 (?1.10, ?0.44) | ?0.46 (?0.79, ?0.14) | ?0.31 (?0.63, 0.01) | |
| GAD-7 | base line | 15.31 | 4.50 | 15.36 | 4.72 | 15.98 | 4.66 | 0.510 | ?0.15 (?0.47, 0.17) | ?0.14 (?0.45, 0.18) | ?0.01(?0.33, 0.31) |
| post-test | 12.46 | 3.95 | 14.09 | 4.60 | 15.26 | 3.92 | 7.536** | ?0.62 (?0.95, ?0.30) | ?0.26 (?0.58, 0.06) | ?0.38 (?0.70, ?0.06) | |
| follow up | 12.71 | 4.36 | 13.55 | 4.42 | 15.23 | 3.75 | 6.103** | ?0.56 (?0.88, ?0.24) | ?0.37 (?0.69, ?0.05) | ?0.19 (?051, 0.12) | |
Table 1 Tests of variance and intervention effect sizes based on intention-to-treat analysis
| outcome variable | Intervention group (n = 76) | Control group (n = 76) | Waiting group (n = 76) | Difference between groups | Intergroup effect sizes (Cohen's d, 95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F (2, 671) | Intervention group vs. waiting group | Control Group vs. Waiting Group | Intervention group vs. control group | ||
| SHAI | base line | 27.64 | 8.53 | 28.20 | 9.95 | 29.45 | 8.75 | 0.852 | ?0.21 (?0.53, 0.11) | ?0.14 (?0.46, 0.17) | ?0.07 (?0.38, 0.25) |
| post-test | 22.41 | 9.03 | 26.64 | 9.41 | 24.27 | 8.51 | 4.767** | ?0.21 (?0.53, 0.11) | 0.27(?0.05, 0.59) | ?0.50 (?0.82, ?0.18) | |
| follow up | 19.98 | 9.14 | 22.17 | 8.92 | 25.11 | 8.69 | 6.574** | ?0.59 (?0.91, ?0.26) | ?0.34 (?0.66, ?0.02) | ?0.26 (?0.58, 0.06) | |
| CABAH | base line | 36.54 | 6.07 | 36.79 | 6.40 | 35.00 | 6.31 | 2.343 | 0.28(?0.04, 0.60) | 0.33 (0.01, 0.65) | ?0.05 (?0.36, 0.27) |
| post-test | 29.22 | 6.74 | 32.22 | 6.47 | 33.77 | 5.60 | 13.835*** | ?0.83 (?1.16, ?0.50) | ?0.28 (?0.60, 0.04) | ?0.56 (?0.89, ?0.24) | |
| follow up | 29.87 | 7.30 | 32.48 | 6.20 | 34.05 | 5.97 | 11.471*** | ?0.76 (?1.09, ?0.44) | ?0.29 (?0.61, 0.03) | ?0.49 (?0.81, ?0.17) | |
| PHQ-9 | base line | 19.12 | 4.95 | 19.80 | 5.71 | 20.33 | 4.97 | 1.063 | -0.23 (?0.55, 0.08) | ?0.10 (?0.42, 0.22) | ?0.14 (?0.45, 0.18) |
| post-test | 15.79 | 4.12 | 17.29 | 5.46 | 20.59 | 4.74 | 17.244*** | ?0.94 (?1.27, ?0.60) | ?0.64 (?0.97, ?0.32) | ?0.30 (?0.62, 0.02) | |
| follow up | 16.72 | 4.35 | 18.26 | 5.75 | 20.64 | 4.10 | 11.212*** | ?0.77 (?1.10, ?0.44) | ?0.46 (?0.79, ?0.14) | ?0.31 (?0.63, 0.01) | |
| GAD-7 | base line | 15.31 | 4.50 | 15.36 | 4.72 | 15.98 | 4.66 | 0.510 | ?0.15 (?0.47, 0.17) | ?0.14 (?0.45, 0.18) | ?0.01(?0.33, 0.31) |
| post-test | 12.46 | 3.95 | 14.09 | 4.60 | 15.26 | 3.92 | 7.536** | ?0.62 (?0.95, ?0.30) | ?0.26 (?0.58, 0.06) | ?0.38 (?0.70, ?0.06) | |
| follow up | 12.71 | 4.36 | 13.55 | 4.42 | 15.23 | 3.75 | 6.103** | ?0.56 (?0.88, ?0.24) | ?0.37 (?0.69, ?0.05) | ?0.19 (?051, 0.12) | |
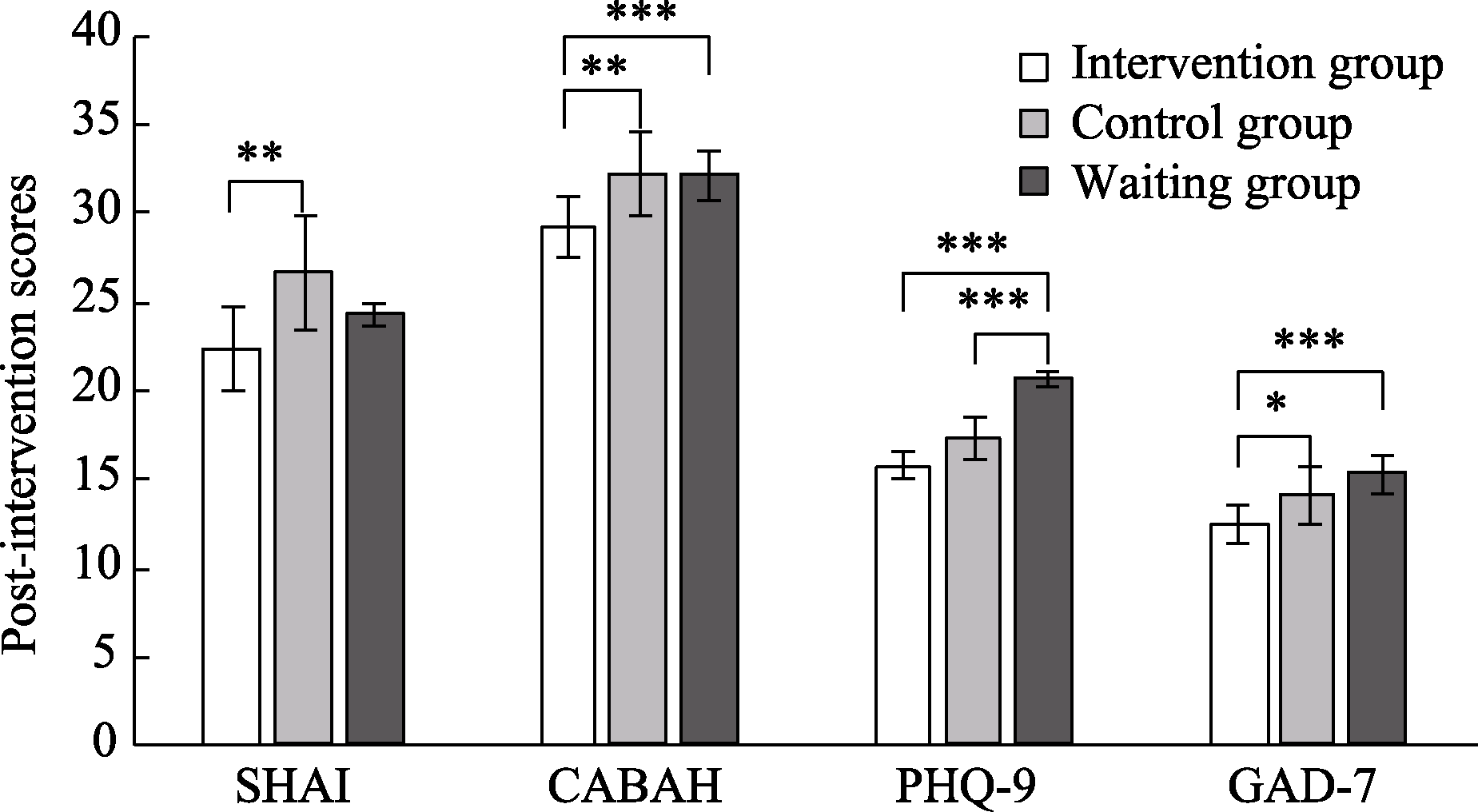
Figure 2. Differences between groups after intervention. Note. SHAI: Short Form Health Anxiety Inventory; CABAH: Body and Health Awareness Scale; PHQ-9: Patient Health Questionnaire; GAD-7: Generalized Anxiety Disorder Scale. * p < 0.05, ** p < 0.01, *** p < 0.001
| Model | χ2 (df) | χ2 /df | SRMR | CFI | AIC | BIC |
|---|---|---|---|---|---|---|
| Reference | — | < 3 | < 0.08 | > 0.95 | — | — |
| SHAI | ||||||
| no-growth model | 98.29(13)*** | 7.56 | 0.25 | 0.82 | 4886.13 | 4907.29 |
| linear growth model | 49.61(10)*** | 4.96 | 0.14 | 0.92 | 4843.45 | 4873.69 |
| quadratic growth model | 11.26(6) | 1.88 | 0.06 | 0.99 | 4813.09 | 4855.43 |
| Potential base growth model | 33.31(7)*** | 4.76 | 0.04 | 0.95 | 4833.15 | 4872.46 |
| CABAH | ||||||
| no-growth model | 96.27(13)*** | 7.41 | 0.27 | 0.84 | 4308.65 | 4329.82 |
| linear growth model | 28.63(10)** | 2.86 | 0.11 | 0.96 | 4247.01 | 4277.25 |
| quadratic growth model | 10.24(6) | 1.71 | 0.05 | 0.99 | 4236.61 | 4278.95 |
| Potential base growth model | 14.70(7)* | 2.10 | 0.03 | 0.99 | 4239.08 | 4278.39 |
Table 2 SHAI and CABAH change trajectory test metrics
| Model | χ2 (df) | χ2 /df | SRMR | CFI | AIC | BIC |
|---|---|---|---|---|---|---|
| Reference | — | < 3 | < 0.08 | > 0.95 | — | — |
| SHAI | ||||||
| no-growth model | 98.29(13)*** | 7.56 | 0.25 | 0.82 | 4886.13 | 4907.29 |
| linear growth model | 49.61(10)*** | 4.96 | 0.14 | 0.92 | 4843.45 | 4873.69 |
| quadratic growth model | 11.26(6) | 1.88 | 0.06 | 0.99 | 4813.09 | 4855.43 |
| Potential base growth model | 33.31(7)*** | 4.76 | 0.04 | 0.95 | 4833.15 | 4872.46 |
| CABAH | ||||||
| no-growth model | 96.27(13)*** | 7.41 | 0.27 | 0.84 | 4308.65 | 4329.82 |
| linear growth model | 28.63(10)** | 2.86 | 0.11 | 0.96 | 4247.01 | 4277.25 |
| quadratic growth model | 10.24(6) | 1.71 | 0.05 | 0.99 | 4236.61 | 4278.95 |
| Potential base growth model | 14.70(7)* | 2.10 | 0.03 | 0.99 | 4239.08 | 4278.39 |
| Paragraph/heading | entry number | Description of checklist entries | pagination |
|---|---|---|---|
| Title and summary | |||
| 1a | A trial that can be identified as randomized in the title of the text | p1 | |
| 1b | Summarize the experimental design, methods, results and conclusions in a structured abstract | p1 | |
| introduction | |||
| Background and purpose | 2a | Explanation of the scientific background and rationale of the research topic | p2-4 |
| 2b | Specific purpose or hypothesis of the research topic | p5 | |
| methods | |||
| Experimental design | 3a | Describe experimental designs that include allocation ratios (e.g., parallel designs, factorial designs) | p5 |
| 3b | Significant changes in methodology and rationale (e.g., eligibility criteria) after test initiation | NA | |
| participant (in a clinical trial etc) | 4a | Eligibility criteria for participants | p5 |
| 4b | Environment and location of data collection | p5 | |
| Methods of intervention | 5 | Details of the interventions in each group and how and when they were actually implemented in order to repeat the trial | p6-8 |
| outcome indicator | 6a | Clear definition of pre-established primary and secondary outcome indicators, including methodology and timing of measurement | p5-6 |
| 6b | Any change in test outcome after test initiation and the rationale for it | NA | |
| sample size | 7a | How the sample size was determined | p5 |
| 7b | Any interim analyses should be explained and the principle of termination of the trial should be given. | NA | |
| Random Sequence Generation | 8a | Methods used to generate randomized allocation order | p8 |
| 8b | Type of randomization, any qualifying details (e.g., block grouping and sample size for each block group) | p8 | |
| Assignment hiding | 9 | Methods used to implement a randomized allocation order (e.g. sequentially numbered containers), describing the steps taken to hide the order prior to the allocation intervention | p8 |
| realize | 10 | Who generated the order of assignment, who enrolled subjects, who assigned subjects to intervention groups | p8 |
| masking (in scientific experiments) | 11a | If blinding was used, assign who was blinded after the intervention (e.g., subjects, health care providers, and outcome assessors) | p8 |
| 11b | Describe the similarity of interventions | p6-7 | |
| Statistical methods | 12a | Statistical methods used to compare primary and secondary outcomes across groups | p8-9 |
| 12b | Additional analytical methods, such as subgroup analysis and calibration analysis | p9 | |
| results | |||
| Subject inclusion process | 13a | Number of people in each group who were randomly assigned, received the intended treatment, and analyzed for the primary outcome | p7-8 |
| 13b | Losses and exclusions after randomization and reasons for each group | NA | |
| Recruitment | 14a | Use dates to clarify recruitment and follow-up times | p7-8 |
| 14b | Why is the experiment over or suspended? | NA | |
| Baseline data | 15 | Tables showing baseline demographics and clinical characteristics of the groups | p25-26 |
| Number of subjects included in the analysis | 16 | Analyze the number of subjects included in each group at a time (denominator), regardless of whether the original subgroups were used. | p25-26 |
| Estimates of outcomes and effects | 17a | Each primary and secondary outcome result for each group, estimated effect sizes and their precision (e.g., 95% confidence intervals) | p9-12 |
| 17b | For both categorical outcomes, both absolute and relative effect sizes are recommended. | p9-12 | |
| complementary analysis | 18 | Report any other analyses performed, including subgroup analyses, corrected analyses, and distinguish which were intended? Which were exploratory? | p12-14 |
| adverse reaction | 19 | All significant hazards or unintended effects for each group | NA |
| discussion | |||
| limitations | 20 | Limitations of the test, suggesting sources of potential bias, lack of precision, and possibly diversity of analyses | p16-17 |
| replicability | 21 | Generalizability of test results (external validity, applicability) | p16 |
| account for | 22 | Provide explanations that are consistent with the results, balance the benefits and harms, and consider other relevant evidence. | p14-16 |
| Other information | |||
| enrollment | 23 | Registration number and name of the test registration | p5 |
| Research program | 24 | Where to access the full pilot program when needed | p5 |
| Funding | 25 | Sources of funding and other support (e.g., drug supply), role of funders | NA |
Table 1 2010 Edition CONSORT Statement - Project Checklist for Reporting Parallel Group Randomized Trials
| Paragraph/heading | entry number | Description of checklist entries | pagination |
|---|---|---|---|
| Title and summary | |||
| 1a | A trial that can be identified as randomized in the title of the text | p1 | |
| 1b | Summarize the experimental design, methods, results and conclusions in a structured abstract | p1 | |
| introduction | |||
| Background and purpose | 2a | Explanation of the scientific background and rationale of the research topic | p2-4 |
| 2b | Specific purpose or hypothesis of the research topic | p5 | |
| methods | |||
| Experimental design | 3a | Describe experimental designs that include allocation ratios (e.g., parallel designs, factorial designs) | p5 |
| 3b | Significant changes in methodology and rationale (e.g., eligibility criteria) after test initiation | NA | |
| participant (in a clinical trial etc) | 4a | Eligibility criteria for participants | p5 |
| 4b | Environment and location of data collection | p5 | |
| Methods of intervention | 5 | Details of the interventions in each group and how and when they were actually implemented in order to repeat the trial | p6-8 |
| outcome indicator | 6a | Clear definition of pre-established primary and secondary outcome indicators, including methodology and timing of measurement | p5-6 |
| 6b | Any change in test outcome after test initiation and the rationale for it | NA | |
| sample size | 7a | How the sample size was determined | p5 |
| 7b | Any interim analyses should be explained and the principle of termination of the trial should be given. | NA | |
| Random Sequence Generation | 8a | Methods used to generate randomized allocation order | p8 |
| 8b | Type of randomization, any qualifying details (e.g., block grouping and sample size for each block group) | p8 | |
| Assignment hiding | 9 | Methods used to implement a randomized allocation order (e.g. sequentially numbered containers), describing the steps taken to hide the order prior to the allocation intervention | p8 |
| realize | 10 | Who generated the order of assignment, who enrolled subjects, who assigned subjects to intervention groups | p8 |
| masking (in scientific experiments) | 11a | If blinding was used, assign who was blinded after the intervention (e.g., subjects, health care providers, and outcome assessors) | p8 |
| 11b | Describe the similarity of interventions | p6-7 | |
| Statistical methods | 12a | Statistical methods used to compare primary and secondary outcomes across groups | p8-9 |
| 12b | Additional analytical methods, such as subgroup analysis and calibration analysis | p9 | |
| results | |||
| Subject inclusion process | 13a | Number of people in each group who were randomly assigned, received the intended treatment, and analyzed for the primary outcome | p7-8 |
| 13b | Losses and exclusions after randomization and reasons for each group | NA | |
| Recruitment | 14a | Use dates to clarify recruitment and follow-up times | p7-8 |
| 14b | Why is the experiment over or suspended? | NA | |
| Baseline data | 15 | Tables showing baseline demographics and clinical characteristics of the groups | p25-26 |
| Number of subjects included in the analysis | 16 | Analyze the number of subjects included in each group at a time (denominator), regardless of whether the original subgroups were used. | p25-26 |
| Estimates of outcomes and effects | 17a | Each primary and secondary outcome result for each group, estimated effect sizes and their precision (e.g., 95% confidence intervals) | p9-12 |
| 17b | For both categorical outcomes, both absolute and relative effect sizes are recommended. | p9-12 | |
| complementary analysis | 18 | Report any other analyses performed, including subgroup analyses, corrected analyses, and distinguish which were intended? Which were exploratory? | p12-14 |
| adverse reaction | 19 | All significant hazards or unintended effects for each group | NA |
| discussion | |||
| limitations | 20 | Limitations of the test, suggesting sources of potential bias, lack of precision, and possibly diversity of analyses | p16-17 |
| replicability | 21 | Generalizability of test results (external validity, applicability) | p16 |
| account for | 22 | Provide explanations that are consistent with the results, balance the benefits and harms, and consider other relevant evidence. | p14-16 |
| Other information | |||
| enrollment | 23 | Registration number and name of the test registration | p5 |
| Research program | 24 | Where to access the full pilot program when needed | p5 |
| Funding | 25 | Sources of funding and other support (e.g., drug supply), role of funders | NA |
| Variable | Intervention group (n = 76) | Control group (n = 76) | Waiting group (n = 76) | Total (n = 228) | F/χ2 | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/N | SD/% | M/N | SD/% | M/N | SD/% | M/N | SD/% | |||||||||
| age | 22.38 | 4.026 | 22.42 | 3.685 | 23.37 | 4.230 | 22.72 | 3.995 | 1.493 | 0.227 | ||||||
| gender | 1.826 | 0.401 | ||||||||||||||
| male | 28 | 36.8% | 33 | 43.4% | 25 | 32.9% | 86 | 37.7% | v | |||||||
| women | 48 | 63.2% | 43 | 56.6% | 51 | 67.1% | 142 | 62.3% | ||||||||
| place of residence | 0.058 | 1.000 | ||||||||||||||
| countryside | 14 | 18.4% | 15 | 19.7% | 14 | 18.4% | 43 | 18.9% | ||||||||
| municipalities | 57 | 75.0% | 56 | 73.7% | 57 | 75.0% | 170 | 74.6% | ||||||||
| suburbia | 5 | 6.6% | 5 | 6.6% | 5 | 6.6% | 15 | 6.6% | ||||||||
| educational level | 15.085 | 0.020 | ||||||||||||||
| High school and below | 3 | 3.9% | 2 | 2.6% | 1 | 1.3% | 6 | 2.6% | ||||||||
| three-year college | 8 | 10.5% | 7 | 9.2% | 3 | 3.9% | 18 | 7.9% | ||||||||
| undergraduate (adjective) | 56 | 73.7% | 58 | 76.3% | 48 | 63.2% | 162 | 71.1% | ||||||||
| Master's degree or above | 9 | 11.8% | 9 | 11.8% | 24 | 31.6% | 42 | 18.4% | ||||||||
| marital status | 0.427 | 0.980 | ||||||||||||||
| Married/cohabiting | 5 | 6.6% | 4 | 5.3% | 5 | 6.6% | 14 | 6.1% | ||||||||
| in love | 24 | 31.6% | 26 | 34.2% | 27 | 35.5% | 77 | 33.8% | ||||||||
| lone | 47 | 61.8% | 46 | 60.5% | 44 | 57.9% | 137 | 60.1% | ||||||||
| working condition | 8.339 | 0.214 | ||||||||||||||
| Full-time work | 18 | 23.7% | 18 | 23.7% | 20 | 26.3% | 56 | 24.6% | ||||||||
| Part-time work | 5 | 6.6% | 4 | 5.3% | 1 | 1.3% | 10 | 4.4% | ||||||||
| No stable job | 2 | 2.6% | 0 | 0 | 5 | 6.6% | 5 | 3.1% | ||||||||
| student at school | 51 | 67.1% | 54 | 71.1% | 50 | 65.8% | 155 | 68.0% | ||||||||
| income status | 5.437 | 0.489 | ||||||||||||||
| Fully satisfied | 10 | 13.2% | 14 | 18.4% | 13 | 17.1% | 37 | 16.2% | ||||||||
| basic necessity | 52 | 68.4% | 51 | 67.1% | 53 | 69.7% | 156 | 68.4% | ||||||||
| largely unsatisfactory | 10 | 13.2% | 9 | 11.8% | 4 | 5.3% | 23 | 10.1% | ||||||||
| income status | Totally unsatisfying. | 4 | 5.3% | 2 | 2.6% | 6 | 7.9% | 12 | 5.3% | |||||||
| symptoms | ||||||||||||||||
| SHAI | 27.93 | 8.53 | 28.79 | 9.95 | 28.25 | 8.75 | 28.32 | 9.07 | 0.172 | 0.842 | ||||||
| CABAH | 36.91 | 6.07 | 37.49 | 6.40 | 33.36 | 6.31 | 35.92 | 6.50 | 9.702 | < 0.001 | ||||||
| PHQ-9 | 18.96 | 4.95 | 19.67 | 5.71 | 19.61 | 4.97 | 19.41 | 5.21 | 0.429 | 0.651 | ||||||
| GAD-7 | 15.62 | 4.50 | 15.75 | 4.72 | 16.14 | 4.66 | 15.84 | 4.61 | 0.266 | 0.766 | ||||||
Table 2 Basic Characteristics of the Sample
| Variable | Intervention group (n = 76) | Control group (n = 76) | Waiting group (n = 76) | Total (n = 228) | F/χ2 | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/N | SD/% | M/N | SD/% | M/N | SD/% | M/N | SD/% | |||||||||
| age | 22.38 | 4.026 | 22.42 | 3.685 | 23.37 | 4.230 | 22.72 | 3.995 | 1.493 | 0.227 | ||||||
| gender | 1.826 | 0.401 | ||||||||||||||
| male | 28 | 36.8% | 33 | 43.4% | 25 | 32.9% | 86 | 37.7% | v | |||||||
| women | 48 | 63.2% | 43 | 56.6% | 51 | 67.1% | 142 | 62.3% | ||||||||
| place of residence | 0.058 | 1.000 | ||||||||||||||
| countryside | 14 | 18.4% | 15 | 19.7% | 14 | 18.4% | 43 | 18.9% | ||||||||
| municipalities | 57 | 75.0% | 56 | 73.7% | 57 | 75.0% | 170 | 74.6% | ||||||||
| suburbia | 5 | 6.6% | 5 | 6.6% | 5 | 6.6% | 15 | 6.6% | ||||||||
| educational level | 15.085 | 0.020 | ||||||||||||||
| High school and below | 3 | 3.9% | 2 | 2.6% | 1 | 1.3% | 6 | 2.6% | ||||||||
| three-year college | 8 | 10.5% | 7 | 9.2% | 3 | 3.9% | 18 | 7.9% | ||||||||
| undergraduate (adjective) | 56 | 73.7% | 58 | 76.3% | 48 | 63.2% | 162 | 71.1% | ||||||||
| Master's degree or above | 9 | 11.8% | 9 | 11.8% | 24 | 31.6% | 42 | 18.4% | ||||||||
| marital status | 0.427 | 0.980 | ||||||||||||||
| Married/cohabiting | 5 | 6.6% | 4 | 5.3% | 5 | 6.6% | 14 | 6.1% | ||||||||
| in love | 24 | 31.6% | 26 | 34.2% | 27 | 35.5% | 77 | 33.8% | ||||||||
| lone | 47 | 61.8% | 46 | 60.5% | 44 | 57.9% | 137 | 60.1% | ||||||||
| working condition | 8.339 | 0.214 | ||||||||||||||
| Full-time work | 18 | 23.7% | 18 | 23.7% | 20 | 26.3% | 56 | 24.6% | ||||||||
| Part-time work | 5 | 6.6% | 4 | 5.3% | 1 | 1.3% | 10 | 4.4% | ||||||||
| No stable job | 2 | 2.6% | 0 | 0 | 5 | 6.6% | 5 | 3.1% | ||||||||
| student at school | 51 | 67.1% | 54 | 71.1% | 50 | 65.8% | 155 | 68.0% | ||||||||
| income status | 5.437 | 0.489 | ||||||||||||||
| Fully satisfied | 10 | 13.2% | 14 | 18.4% | 13 | 17.1% | 37 | 16.2% | ||||||||
| basic necessity | 52 | 68.4% | 51 | 67.1% | 53 | 69.7% | 156 | 68.4% | ||||||||
| largely unsatisfactory | 10 | 13.2% | 9 | 11.8% | 4 | 5.3% | 23 | 10.1% | ||||||||
| income status | Totally unsatisfying. | 4 | 5.3% | 2 | 2.6% | 6 | 7.9% | 12 | 5.3% | |||||||
| symptoms | ||||||||||||||||
| SHAI | 27.93 | 8.53 | 28.79 | 9.95 | 28.25 | 8.75 | 28.32 | 9.07 | 0.172 | 0.842 | ||||||
| CABAH | 36.91 | 6.07 | 37.49 | 6.40 | 33.36 | 6.31 | 35.92 | 6.50 | 9.702 | < 0.001 | ||||||
| PHQ-9 | 18.96 | 4.95 | 19.67 | 5.71 | 19.61 | 4.97 | 19.41 | 5.21 | 0.429 | 0.651 | ||||||
| GAD-7 | 15.62 | 4.50 | 15.75 | 4.72 | 16.14 | 4.66 | 15.84 | 4.61 | 0.266 | 0.766 | ||||||
| Variable | F | df | p |
|---|---|---|---|
| gender | 2.158 | (1, 220) | 0.143 |
| group | 5.313 | (2, 220) | 0.006 |
| gender × group | 1.247 | (2, 220) | 0.290 |
| place of residence | 1.057 | (2, 217) | 0.349 |
| group | 4.585 | (2, 217) | 0.011 |
| place of residence × group | 0.345 | (2, 217) | 0.847 |
| educational level | 1.244 | (2, 218) | 0.290 |
| group | 2.526 | (2, 218) | 0.082 |
| educational level × group | 0.414 | (2, 218) | 0.798 |
| marital status | 0.213 | (2, 217) | 0.808 |
| group | 6.529 | (2, 217) | 0.002 |
| marital status × group | 1.959 | (2, 217) | 0.102 |
| working condition | 0.133 | (2, 217) | 0.876 |
| group | 1.890 | (2, 217) | 0.154 |
| working status × group | 1.965 | (4, 217) | 0.101 |
| Income status | 0.691 | (2, 217) | 0.502 |
| group | 4.304 | (2, 217) | 0.015 |
| Income status × group | 1.429 | (4, 217) | 0.225 |
Table 3.1 Demographic Categorical Variables and Groups ANOVA
| Variable | F | df | p |
|---|---|---|---|
| gender | 2.158 | (1, 220) | 0.143 |
| group | 5.313 | (2, 220) | 0.006 |
| gender × group | 1.247 | (2, 220) | 0.290 |
| place of residence | 1.057 | (2, 217) | 0.349 |
| group | 4.585 | (2, 217) | 0.011 |
| place of residence × group | 0.345 | (2, 217) | 0.847 |
| educational level | 1.244 | (2, 218) | 0.290 |
| group | 2.526 | (2, 218) | 0.082 |
| educational level × group | 0.414 | (2, 218) | 0.798 |
| marital status | 0.213 | (2, 217) | 0.808 |
| group | 6.529 | (2, 217) | 0.002 |
| marital status × group | 1.959 | (2, 217) | 0.102 |
| working condition | 0.133 | (2, 217) | 0.876 |
| group | 1.890 | (2, 217) | 0.154 |
| working status × group | 1.965 | (4, 217) | 0.101 |
| Income status | 0.691 | (2, 217) | 0.502 |
| group | 4.304 | (2, 217) | 0.015 |
| Income status × group | 1.429 | (4, 217) | 0.225 |
| variable | β | t | ΔR2 | F | |
|---|---|---|---|---|---|
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | ?0.168 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.051 | 2.719* | |||
| D1 | 0.229 | 3.041** | |||
| D2 | 0.011 | 0.139 | |||
| age | ?0.038 | ?0.574 | |||
| third step | 0.003 | 2.029 | |||
| D1 x age | ?0.072 | ?0.789 | |||
| D2 x age | ?0.059 | ?0.619 | |||
| initial step | 0.002 | 0.410 | |||
| educational level | 0.043 | 0.640 | |||
| second step | 0.054 | 3.326* | |||
| D1 | 0.229 | 3.047 | |||
| D2 | 0.007 | 0.093 | |||
| CABAH baseline level | 0.037 | 0.544 | |||
| third step | 0.021 | 3.091** | |||
| D1 x CABAH baseline level | 0.017 | 0.178 | |||
| D2 x CABAH baseline level | 0.197 | 2.006* | |||
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | 0.703 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.051 | 2.709* | |||
| D1 | 0.230 | 3.052** | |||
| D2 | 0.014 | 0.177 | |||
| SHAI baseline level | ?0.042 | ?0.533 | |||
| third step | 0.054 | 3.955*** | |||
| D1 x SHAI baseline level | 0.070 | 0.715 | |||
| D2 x SHAI baseline level | 0.311 | 3.405** | |||
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | 0.703 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.085 | 4.492** | |||
| D1 | 0.230 | 3.105** | |||
| D2 | ?0.014 | ?0.175 | |||
| GAD-7 baseline level | 0.198 | 2.949 | |||
| third step | 0.054 | 3.955*** | |||
| D1 x GAD-7 baseline level | 0.061 | 0.658 | |||
| D2 x GAD-7 baseline level | 0.126 | 1.351 | |||
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | 0.703 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.075 | 3.984*** | |||
| D1 | 0.220 | 2.957 | |||
| D2 | ?0.019 | ?0.237 | |||
| PHQ-9 baseline level | 0.169 | 2.510* | |||
| third step | 0.011 | 3.209*** | |||
| D1 x PHQ-9 baseline level | 0.050 | 0.505 | |||
| D2 x PHQ-9 baseline level | 0.140 | 1.536 |
Table 3.2 Stratified Regression Analysis of Age, Baseline Level of Symptoms, and Groups
| variable | β | t | ΔR2 | F | |
|---|---|---|---|---|---|
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | ?0.168 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.051 | 2.719* | |||
| D1 | 0.229 | 3.041** | |||
| D2 | 0.011 | 0.139 | |||
| age | ?0.038 | ?0.574 | |||
| third step | 0.003 | 2.029 | |||
| D1 x age | ?0.072 | ?0.789 | |||
| D2 x age | ?0.059 | ?0.619 | |||
| initial step | 0.002 | 0.410 | |||
| educational level | 0.043 | 0.640 | |||
| second step | 0.054 | 3.326* | |||
| D1 | 0.229 | 3.047 | |||
| D2 | 0.007 | 0.093 | |||
| CABAH baseline level | 0.037 | 0.544 | |||
| third step | 0.021 | 3.091** | |||
| D1 x CABAH baseline level | 0.017 | 0.178 | |||
| D2 x CABAH baseline level | 0.197 | 2.006* | |||
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | 0.703 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.051 | 2.709* | |||
| D1 | 0.230 | 3.052** | |||
| D2 | 0.014 | 0.177 | |||
| SHAI baseline level | ?0.042 | ?0.533 | |||
| third step | 0.054 | 3.955*** | |||
| D1 x SHAI baseline level | 0.070 | 0.715 | |||
| D2 x SHAI baseline level | 0.311 | 3.405** | |||
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | 0.703 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.085 | 4.492** | |||
| D1 | 0.230 | 3.105** | |||
| D2 | ?0.014 | ?0.175 | |||
| GAD-7 baseline level | 0.198 | 2.949 | |||
| third step | 0.054 | 3.955*** | |||
| D1 x GAD-7 baseline level | 0.061 | 0.658 | |||
| D2 x GAD-7 baseline level | 0.126 | 1.351 | |||
| initial step | 0.007 | 0.808 | |||
| educational level | 0.047 | 0.703 | |||
| CABAH baseline level | 0.073 | 1.098 | |||
| second step | 0.075 | 3.984*** | |||
| D1 | 0.220 | 2.957 | |||
| D2 | ?0.019 | ?0.237 | |||
| PHQ-9 baseline level | 0.169 | 2.510* | |||
| third step | 0.011 | 3.209*** | |||
| D1 x PHQ-9 baseline level | 0.050 | 0.505 | |||
| D2 x PHQ-9 baseline level | 0.140 | 1.536 |
| [1] |
Agarwal, G., Varghese, S., Francis, M., & Willan, J. (2023). High prevalence of persistent COVID-19-related health anxiety and social restriction in patients with haematological disorders. British Journal of Haematology, 202(5), 1065-1070.
doi: 10.1111/bjh.18960 pmid: 37408108 |
| [2] | Akbari, M., Spada, M. M., Nikčević, A. V., & Zamani, E. (2021). The relationship between fear of covid-19 and health anxiety among families with covid-19 infected: The mediating role of metacognitions, intolerance of uncertainty and emotion regulation. Clinical Psychology & Psychotherapy, 28(6), 1354-1366. |
| [3] | Antognelli, S. L., Sharrock, M. J., & Newby, J. M. (2020). A randomised controlled trial of computerised interpretation bias modification for health anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 66, 101518. |
| [4] | Aue, T., & Okon-Singer, H. (2020). Cognitive biases in health and psychiatric disorders: Neurophysiological foundations. Academic Press. |
| [5] |
Axelsson, E., Andersson, E., Ljótsson, B., Björkander, D., Hedman-Lagerlöf, M., & Hedman-Lagerlöf, E. (2020). Effect of internet vs face-to-face cognitive behavior therapy for health anxiety: A randomized noninferiority clinical trial. JAMA Psychiatry, 77(9), 915-924.
doi: 10.1001/jamapsychiatry.2020.0940 pmid: 32401286 |
| [6] |
Bailey, R., & Wells, A. (2015). Metacognitive beliefs moderate the relationship between catastrophic misinterpretation and health anxiety. Journal of Anxiety Disorders, 34, 8-14.
doi: 10.1016/j.janxdis.2015.05.005 pmid: 26093824 |
| [7] | Bredemeier, K., Church, L., Bounoua, N., Feler, B., & Spielberg, J. M. (2023). Intolerance of uncertainty, anxiety sensitivity, and health anxiety during the COVID-19 pandemic: Exploring temporal relationships using cross-lag analysis. Journal of Anxiety Disorders, 93, 102660. |
| [8] |
Brown, T. A. (2007). Temporal course and structural relationships among dimensions of temperament and DSM-IV anxiety and mood disorder constructs. Journal of Abnormal Psychology, 116(2), 313-328.
pmid: 17516764 |
| [9] |
Bults, M., Beaujean, D. J., de Zwart, O., Kok, G., van Empelen, P., van Steenbergen, J. E.,... Voeten, H. A. (2011). Perceived risk, anxiety, and behavioural responses of the general public during the early phase of the Influenza A (H1N1) pandemic in the Netherlands: Results of three consecutive online surveys. BMC Public Health, 11, 2.
doi: 10.1186/1471-2458-11-2 pmid: 21199571 |
| [10] | Burnham, K. P., & Anderson, D. R. (2004). Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33(2), 261-304. |
| [11] | Capron, D. W., Norr, A. M., Allan, N. P., & Schmidt, N. B. (2017). Combined “top-down” and “bottom-up” intervention for anxiety sensitivity: Pilot randomized trial testing the additive effect of interpretation bias modification. Journal of Psychiatric Research, 85, 75-82. |
| [12] | Chan, F. H. F., Takano, K., Lau, J. Y. F., & Barry, T. J. (2020). Evaluation of the factor structure and content specificity of the interpretation bias task (IBT). Cognitive Therapy and Research, 44(6), 1213-1224. |
| [13] |
Clark, D., Beck, A., & Brown, G. (1989). Cognitive mediation in general psychiatric outpatients: A test of the content-specificity hypothesis. Journal of Personality and Social Psychology, 56(6), 958-964.
pmid: 2746459 |
| [14] |
Dezutter, J., Luyckx, K., Schaap-Jonker, H., Büssing, A., Corveleyn, J., & Hutsebaut, D. (2010). God image and happiness in chronic pain patients: The mediating role of disease interpretation. Pain medicine, 11(5), 765-773.
doi: 10.1111/j.1526-4637.2010.00827.x pmid: 20353410 |
| [15] | Dreier, M., Ludwig, J., Hrter, M., Knesebeck, O. V. D., Baumgardt, J., Bock, T.,... Liebherz, S. (2019). Development and evaluation of e-mental health interventions to reduce stigmatization of suicidality: A study protocol. BMC Psychiatry, 19(1), 152. |
| [16] | Du, N., Yu, K., Ye, Y., & Chen, S. (2017). Validity study of Patient Health Questionnaire-9 items for Internet screening in depression among Chinese university students. Asia-Pacific Psychiatry, 9(3), e12266. |
| [17] | Du, X., Witthöft, M., Zhang, T., Shi, C., & Ren, Z. (2023). Interpretation bias in health anxiety: A systematic review and meta-analysis. Psychological Medicine, 53(1), 34-45. |
| [18] |
Eilenberg, T., Hoffmann, D., Jensen, J. S., & Frostholm, L. (2017). Intervening variables in group-based acceptance & commitment therapy for severe health anxiety. Behaviour Research and Therapy, 92, 24-31.
doi: S0005-7967(17)30016-5 pmid: 28196772 |
| [19] |
Falkenstein, M., Kelley, K., Dattolico, D., Kuckertz, J., Bezahler, A., Krompinger, J., Webb, C., & Beard, C. (2022). Feasibility and acceptability of cognitive bias modification for interpretation as an adjunctive treatment for OCD and related disorders: A pilot randomized controlled trial. Behavior Therapy, 53(2), 294-309.
doi: 10.1016/j.beth.2021.09.002 pmid: 35227405 |
| [20] | Fodor, L. A., Georgescu, R., Cuijpers, P., Szamoskozi, Ş., David, D., Furukawa, T. A., & Cristea, I. A. (2020). Efficacy of cognitive bias modification interventions in anxiety and depressive disorders: A systematic review and network meta-analysis. The Lancet Psychiatry, 7(6), 506-514. |
| [21] | Frazier, P. A., Tix, A. P., & Barron, K. E. (2004). Testing moderator and mediator effects in counseling psychology research. Journal of Counseling Psychology, 51(1), 115-134. |
| [22] | Gellatly, R., & Beck, A. T. (2016). Catastrophic thinking: A transdiagnostic process across psychiatric disorders. Cognitive Therapy and Research, 40(4), 441-452. |
| [23] |
Gong, Y., Zhou, H., Zhang, Y., Zhu, X., Wang, X., Shen, B., Xian, J., & Ding, Y. (2021). Validation of the 7-item Generalized Anxiety Disorder scale (GAD-7) as a screening tool for anxiety among pregnant Chinese women. Journal of Affective Disorders, 282, 98-103.
doi: 10.1016/j.jad.2020.12.129 pmid: 33401129 |
| [24] | Hedman, E., Axelsson, E., Gorling, A., Ritzman, C., Ronnheden, M., El Alaoui, S.,... Ljótsson, B. (2014). Internet-delivered exposure-based cognitive-behavioural therapy and behavioural stress management for severe health anxiety: Randomised controlled trial. The British Journal of Psychiatry, 205(4), 307-314. |
| [25] | Hedman-Lagerlöf, E., Tyrer, P., Hague, J., & Tyrer, H. (2019). Health anxiety. BMJ, 364, l774. |
| [26] |
Helmich, M. A., Wichers, M., Olthof, M., Strunk, G., Aas, B., Aichhorn, W., Schiepek, G., & Snippe, E. (2020). Sudden gains in day-to-day change: Revealing nonlinear patterns of individual improvement in depression. Journal of Consulting and Clinical Psychology, 88(2), 119-127.
doi: 10.1037/ccp0000469 pmid: 31894994 |
| [27] |
Hirsch, C. R., Krahé, C., Whyte, J., Krzyzanowski, H., Meeten, F., Norton, S., & Mathews, A. (2021). Internet-delivered interpretation training reduces worry and anxiety in individuals with generalized anxiety disorder: A randomized controlled experiment. Journal of Consulting and Clinical Psychology, 89(7), 575-589.
doi: 10.1037/ccp0000660 pmid: 34383532 |
| [28] | Hirsch, C. R., Meeten, F., Krahé, C., & Reeder, C. (2016). Resolving ambiguity in emotional disorders: The nature and role of interpretation biases. Annual Review of Clinical Psychology, 12(1), 281-305. |
| [29] | Hoffmann, D., Rask, C. U., Hedman-Lagerlöf, E., Jensen, J. S., & Frostholm, L. (2021). Efficacy of internet-delivered acceptance and commitment therapy for severe health anxiety: Results from a randomized, controlled trial. Psychological Medicine, 51(15), 2685-2695. |
| [30] | Hu, L., & Bentler, P. M. (1998). Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods, 3(4), 424-453. |
| [31] | Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1-55. |
| [32] | Jalloh, M. F., Li, W., Bunnell, R. E., Ethier, K. A., O' Leary, A., Hageman, K. M.,... Redd, J. T. (2018). Impact of Ebola experiences and risk perceptions on mental health in Sierra Leone, July 2015. BMJ Global Health, 3(2), e000471. |
| [33] | Ji, J. L., Baee, S., Zhang, D., Calicho-Mamani, C. P., Meyer, M. J., Funk, D., … Teachman, B. A. (2021). Multi-session online interpretation bias training for anxiety in a community sample. Behaviour Research and Therapy, 142, 103864. |
| [34] |
Jones, E. B., & Sharpe, L. (2017). Cognitive bias modification: A review of meta-analyses. Journal of Affective Disorders, 223, 175-183.
doi: S0165-0327(17)31096-0 pmid: 28759865 |
| [35] | Kerstner, T., Witthöft, M., Mier, D., Diener, C., Rist, F., & Bailer, J. (2015). A diary-based modification of symptom attributions in pathological health anxiety: Effects on symptom report and cognitive biases. Journal of Consulting and Clinical Psychology, 83(3), 578-589. |
| [36] | Kisbu-Sakarya, Y., Mackinnon, D. P., & Aiken, L. S. (2013). A monte carlo comparison study of the power of the analysis of covariance, simple difference, and residual change scores in testing two-wave data. Educational & Psychological Measurement, 73(1), 47-62. |
| [37] | Krebs, G., Pil, V., Grant, S., Esposti, M. D., Montgomery, P., & Lau, J. Y. F. (2018). Research review: Cognitive bias modification of interpretations in youth and its effect on anxiety: A meta-analysis. Journal of Child Psychology and Psychiatry, 59(8), 831-844. |
| [38] | Kroenke, K., & Spitzer, R. L. (2002). The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals, 32(9), 509-515. |
| [39] |
Lau, J. Y. F., Badaoui, M., Meehan, A. J., Heathcote, L. C., Barker, E. D., & Rimes, K. A. (2020). Assessing the content specificity of interpretation biases in community adolescents with persistent and interfering pain. Pain, 161(2), 319-327.
doi: 10.1097/j.pain.0000000000001723 pmid: 31634340 |
| [40] | Liao, S.-C., & Huang, W.-L. (2021). Psychometric properties of the Chinese version of the cognitions about body and health questionnaire. Neuropsychiatric Disease and Treatment, 17, 1135-1144. |
| [41] | Liu, B., & Li, X. (2018). Cognitive bias modification of interpretation for social anxiety: A "bottom-up" intervention? Advances in Psychological Science, 26(5), 859-871. |
| [42] |
Longmore, R. J., & Worrell, M. (2007). Do we need to challenge thoughts in cognitive behavior therapy? Clinical Psychology Review, 27(2), 173-187.
pmid: 17157970 |
| [43] | Luo, J., Wang, P., Li, Z., Cao, W., Liu, H., Meng, L., & Sun, J. (2021). Health anxiety and its correlates in the general Chinese population during the COVID-19 epidemic. Frontiers in Psychiatry, 12, 743409. |
| [44] | MacLeod, C., & Mathews, A. (2012). Cognitive bias modification approaches to anxiety. Annual Review of Clinical Psychology, 8(1), 189-217. |
| [45] | Mahase, E. (2020). China coronavirus: Who declares international emergency as death toll exceeds 200. British Medical Journal, 368, m408. |
| [46] | Main, A., Zhou, Q., Ma, Y., Luecken, L. J., & Liu, X. (2011). Relations of SARS-related stressors and coping to Chinese college students’ psychological adjustment during the 2003 Beijing SARS epidemic. Journal of Counseling Psychology, 58(3), 410-423. |
| [47] |
Mathews, A., & Mackintosh, B. (2000). Induced emotional interpretation bias and anxiety. Journal of Abnormal Psychology, 109(4), 602-615.
pmid: 11195984 |
| [48] |
Mcmanus, F., Surawy, C., Muse, K., Vazquez-Montes, M., & Williams, J. M. G. (2012). A randomized clinical trial of mindfulness-based cognitive therapy versus unrestricted services for health anxiety (hypochondriasis). Journal of Consulting and Clinical Psychology, 80(5), 817-828.
pmid: 22708977 |
| [49] | Menne-Lothmann, C., Viechtbauer, W., Höhn, P., Kasanova, Z., Haller, S. P., Drukker, M.,... Lau, J. Y. F. (2014). How to boost positive interpretations? A meta-analysis of the effectiveness of cognitive bias modification for interpretation. PLoS ONE, 9(6), e100925. |
| [50] | Midi, H., & Bagheri, A. (2010). Robust multicollinearity diagnostic measure in collinear data set. In Proceedings of the 4th international conference on applied mathematics, simulation, modeling (pp. 138-142). World Scientific and Engineering Academy and Society. |
| [51] |
Mobini, S., Mackintosh, B., Illingworth, J., Gega, L., Langdon, P., & Hoppitt, L. (2014). Effects of standard and explicit cognitive bias modification and computer-administered cognitive-behaviour therapy on cognitive biases and social anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 45(2), 272-279.
doi: 10.1016/j.jbtep.2013.12.002 pmid: 24412966 |
| [52] | Morriss, R., Patel, S., Malins, S., Guo, B., Higton, F., James, M.,... Tyrer, H. (2019). Clinical and economic outcomes of remotely delivered cognitive behaviour therapy versus treatment as usual for repeat unscheduled care users with severe health anxiety: A multicentre randomised controlled trial. BMC Medicine, 17(1), 16. |
| [53] | Nieto, I., & Vazquez, C. (2021). Disentangling the mediating role of modifying interpretation bias on emotional distress using a novel cognitive bias modification program. Journal of Anxiety Disorders, 83, 102459. |
| [54] |
Olatunji, B. O., Kauffman, B. Y., Meltzer, S., Davis, M. L., Smits, J. A. J., & Powers, M. B. (2014). Cognitive-behavioral therapy for hypochondriasis/health anxiety: A meta-analysis of treatment outcome and moderators. Behaviour Research and Therapy, 58, 65-74.
doi: 10.1016/j.brat.2014.05.002 pmid: 24954212 |
| [55] |
Ren, Z., Lai, L., Yu, X., Li, S., Ruan, Y., Zhao, L. (2016). Meta-analysis on CBM for anxiety disorder: Effect sizes, moderators and mediation. Advances in Psychological Science, 24(11), 1690-1711.
doi: 10.3724/SP.J.1042.2016.01690 |
| [56] |
Ren, Z., Li, X., Zhao, L., Yu, X., Li, Z., Lai, L., Yuan, Y., & Jiang, G. (2016). Effectiveness and mechanism of internet-based self-help intervention for depression: The Chinese version of MoodGYM. Acta Psychologica Sinica, 48(7), 818-832.
doi: 10.3724/SP.J.1041.2016.00818 |
| [57] |
Ren, Z., Zhao, C., Bian, C., Zhu, W., Jiang, G., Zhu, Z. (2019). Mechanisms of the Acceptance and Commitment Therapy: A meta-analytic structural equation model. Acta Psychologica Sinica, 51(6), 662-676.
doi: 10.3724/SP.J.1041.2019.00662 |
| [58] | Ren, Z., Zhao, Z., Yu, X., Zhang, L., & Li, X. (2021). Modification of hostile interpretation bias and self-reported aggression in juvenile delinquents: A randomized controlled trial. International Journal of Clinical and Health Psychology, 21(2), 100226. |
| [59] |
Rief, W., Hiller, W., & Margraf, J. (1998). Cognitive aspects of hypochondriasis and the somatization syndrome. Journal of Abnormal Psychology, 107(4), 587-595.
pmid: 9830246 |
| [60] | Rios, K., Sosa, N., & Osborn, H. (2018). An experimental approach to Intergroup Threat Theory: Manipulations, moderators, and consequences of realistic vs. symbolic threat. European Review of Social Psychology, 29(1), 212-255. |
| [61] | Rogosa, D. R., Brandt, D., & Zimowski, M. (1982). A growth curve approach to the measurement of change. Psychological Bulletin, 92(3), 726-748. |
| [62] |
Rozenman, M., Gonzalez, A., Logan, C., & Goger, P. (2020). Cognitive bias modification for threat interpretations: Impact on anxiety symptoms and stress reactivity. Depression and Anxiety, 37(5), 438-448.
doi: 10.1002/da.23018 pmid: 32301579 |
| [63] |
Salemink, E., van den Hout, M., & Kindt, M. (2010). How does cognitive bias modification affect anxiety? Mediation analyses and experimental data. Behavioural and Cognitive Psychotherapy, 38(1), 59-66.
doi: 10.1017/S1352465809990543 pmid: 19995465 |
| [64] |
Salkovskis, P. M., & Warwick, H. M. C. (1986). Morbid preoccupations, health anxiety and reassurance: a cognitive-behavioural approach to hypochondriasis. Behaviour Research and Therapy, 24(5), 597-602.
doi: 10.1016/0005-7967(86)90041-0 pmid: 3753387 |
| [65] |
Salkovskis, P. M., Rimes, K. A., Warwick, H. M. C., & Clark, D. M. (2002). The health anxiety inventory: Development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychological Medicine, 32(5), 843-853.
doi: 10.1017/s0033291702005822 pmid: 12171378 |
| [66] | Schulz, K. F., Altman, D. G., Moher, D., & CONSORT, Group. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ, 340, c332-c332. |
| [67] | Selig, J. P., & Preacher, K. J. (2009). Mediation models for longitudinal data in developmental research. Research in human development, 6(2-3), 144-164. |
| [68] |
Sørensen, P., Birket-Smith, M., Wattar, U., Buemann, I., & Salkovskis, P. (2011). A randomized clinical trial of cognitive behavioural therapy versus short-term psychodynamic psychotherapy versus no intervention for patients with hypochondriasis. Psychological Medicine, 41(2), 431-441.
doi: 10.1017/S0033291710000292 pmid: 20380779 |
| [69] |
Spitzer, R. L., Kroenke, K., Williams, J. B. W., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092-1097.
doi: 10.1001/archinte.166.10.1092 pmid: 16717171 |
| [70] | Taylor, S., & Asmundson, G. J. G. (2004). Treating health anxiety: A cognitive-behavioral approach. Guilford Press. |
| [71] | Tolgou, T., Rohrmann, S., Stockhausen, C., Krampen, D., Warnecke, I., & Reiss, N. (2018). Physiological and psychological effects of imagery techniques on health anxiety. Psychophysiology, 55(2), e12984. |
| [72] | Tyrer, P. (2020). COVID-19 health anxiety. World Psychiatry, 19(3), 307-308. |
| [73] | Tyrer, P., Cooper, S., Salkovskis, P., Tyrer, H., Crawford, M., Byford, S.,... Barrett, B. (2014). Clinical and cost-effectiveness of cognitive behaviour therapy for health anxiety in medical patients: A multicentre randomised controlled trial. The Lancet, 383(9913), 219-225. |
| [74] |
van Teffelen, M., Lobbestael, J., Voncken, M., Cougle, J., & Peeters, F. (2021). Interpretation bias modification for hostility: A randomized clinical trial. Journal of Consulting and Clinical Psychology, 89(5), 421-434.
doi: 10.1037/ccp0000651 pmid: 34124926 |
| [75] |
Visser, S., & Bouman, T. K. (2001). The treatment of hypochondriasis: Exposure plus response prevention vs cognitive therapy. Behaviour Research and Therapy, 39(4), 423-442.
pmid: 11280341 |
| [76] | von Soest, T., & Hagtvet, K. A. (2011). Mediation analysis in a latent growth curve modeling framework. Structural Equation Modeling, 18(2), 289-314. |
| [77] |
Walker, J., Vincent, N., Furer, P., Cox, B., & Kevin, Kjernisted. (1999). Treatment preference in hypochondriasis. Journal of Behavior Therapy and Experimental Psychiatry, 30(4), 251-258.
pmid: 10759322 |
| [78] |
Weck, F., Gropalis, M., Hiller, W., & Bleichhardt, G. (2015). Effectiveness of cognitive-behavioral group therapy for patients with hypochondriasis (health anxiety). Journal of Anxiety Disorders, 30, 1-7.
doi: 10.1016/j.janxdis.2014.12.012 pmid: 25589453 |
| [79] |
Weck, F., Neng, J. M. B., Richtberg, S., Jakob, M., & Stangier, U. (2015). Cognitive therapy versus exposure therapy for hypochondriasis (health anxiety): A randomized controlled trial. Journal of Consulting and Clinical Psychology, 83(4), 665-676.
doi: 10.1037/ccp0000013 pmid: 25495359 |
| [80] |
Weck, F., Neng, J. M. B., Schwind, J., & Höfling, V. (2015). Exposure therapy changes dysfunctional evaluations of somatic symptoms in patients with hypochondriasis (health anxiety). A randomized controlled trial. Journal of Anxiety Disorders, 34, 1-7.
doi: 10.1016/j.janxdis.2015.05.008 pmid: 26093823 |
| [81] | Williams, P. G. (2004). The psychopathology of self-assessed health: A cognitive approach to health anxiety and hypochondriasis. Cognitive Therapy and Research, 28(5), 629-644. |
| [82] | Woud, M. L., Wittekind, C. E., & Würtz, F. (2022). Cognitive bias modification bei symptomen der posttraumatischen belastungsstörung. Verhaltenstherapie, 32(3), 139-147. |
| [83] | Yan, Z., Witthöft, M., Bailer, J., Diener, C., & Mier, D. (2019). Scary symptoms? Functional magnetic resonance imaging evidence for symptom interpretation bias in pathological health anxiety. European Archives of Psychiatry and Clinical Neuroscience, 269(4), 195-207. |
| [84] |
Yang, R., Cui, L., Li, F., Xiao, J., Zhang, Q., & Oei, T. P. S. (2017). Effects of cognitive bias modification training via smartphones. Frontiers in Psychology, 8, 1370.
doi: 10.3389/fpsyg.2017.01370 pmid: 28855880 |
| [85] | Yuan, Y., & Zhang, Y. (2013). Health anxiety of patients with chronic disease and its influencing factors. In Psychology and Enhancement of Innovation Capability - Proceedings of the 16th National Academic Congress of Psychology (pp. 2263-2264). Nanjing, China: Chinese Psychological Society. |
| [86] | Zhang, F., Huang, C., Mao, X., Hou, T., Sun, L., Zhou, Y., & Deng, G. (2021). Efficacy of the Chinese version interpretation bias modification training in an unselected sample: A randomized trial. PLOS ONE, 16(7), e0255224. |
| [87] |
Zhang, Y., Liu, R., Li, G., Mao, S., & Yuan, Y. (2015). The reliability and validity of a Chinese-version short health anxiety inventory: An investigation of university students. Neuropsychiatric Disease and Treatment, 11, 1739-1747.
doi: 10.2147/NDT.S83501 pmid: 26213472 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||