1 引言
药物成瘾是一种慢性反复发作的脑疾病, 用药失去控制,高觅药动机以及明知有不良后果仍持续用药是其核心特征(American Psychiatric Association, 2013).停药后个体会出现持久的负性情绪状态.阿片类物质是临床上常用的止痛药, 然而其强烈的奖赏特性能够导致成瘾(Fields & Margolis, 2015).在亚洲, 以娱乐为目的的阿片类物质的使用量仍居首位(World Health Organ, 2010).阿片成瘾人数的不断增长引发了严重的健康,社会和经济问题, 此类药物的过量使用还会致死.因此, 阿片成瘾的神经机制备受关注.
阿片成瘾与中脑边缘多巴胺系统(mesolimbic dopamine system)密切相关(Juarez & Han, 2016).近年来研究发现, 喙内侧被盖核(rostromedial tegmental nucleus, RMTg)是多巴胺系统重要的抑制控制中心, 它在阿片成瘾中的作用受到重视.RMTg位于腹侧被盖区(ventral tegmental area, VTA)的尾部, 也被称为腹侧被盖区尾部(tVTA) (Kaufling, Veinante, Pawlowski, Freund-Mercier, & Barrot, 2009).它包括从VTA的尾状边缘到脚桥被盖核(pedunculopontine tegmental nuclei)的喙状边缘这一区域(Jhou, Geisler, Marinelli, Degarmo, & Zahm, 2009; Kaufling et al., 2009).早期的研究只是把RMTg作为VTA的一部分.后来, 越来越多的研究证据显示, 两者无论是在解剖学上还是在功能上都存在异质性(Sanchez-Catalan, Kaufling, Georges, Veinante, & Barrot, 2014).RMTg富含γ-氨基丁酸(γ-aminobutyric acid, GABA)神经元(>75%) (Kaufling & Aston-Jones, 2015), 缺乏多巴胺能神经元(Jhou, Geisler, et al., 2009).然而, VTA中主要是多巴胺能神经元(55%~65%), 也包含30%左右的GABA能神经元(Pignatelli & Bonci, 2015).RMTg和VTA分别加工厌恶和奖赏刺激, 在调控奖赏环路中发挥相反的作用(Hong, Jhou, Smith, Saleem, & Hikosaka, 2011).因此, RMTg开始被作为一个独立的脑区进行研究.本文首先梳理RMTg与奖赏环路的关系, 尤其是RMTg在负性奖赏环路中的枢纽作用, 然后论述阿片类物质成瘾的机制和以RMTg为靶点治疗阿片成瘾的初期研究.
2 奖赏环路与RMTg
2.1 奖赏环路
奖赏环路是由几个皮层和亚皮层区域构成的一个复杂网络, 参与奖赏加工的各个方面(Haber & Knutson, 2009), 包括编码奖赏效价,奖赏预期错误和奖赏动机凸显等, 并调控奖赏相关行为.奖赏环路可以区分为正性奖赏环路和负性奖赏环路:前者促进奖赏学习及相关行为; 后者编码厌恶信息, 抑制奖赏效应.
2.1.1 正性奖赏环路
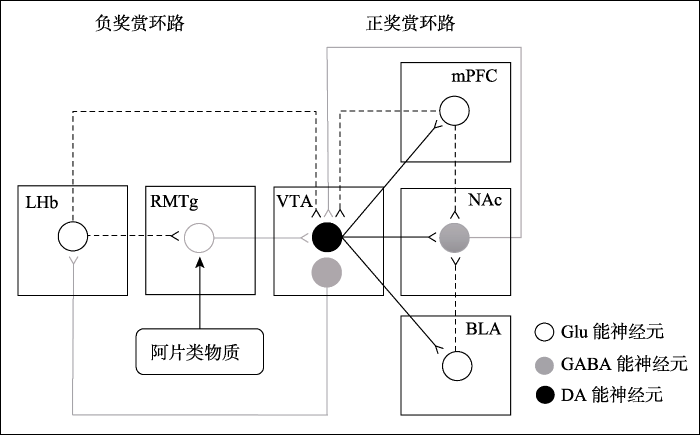
中脑边缘多巴胺系统是奖赏环路的中心(Haber & Knutson, 2009).该系统主要起源于两个脑区:VTA和黑质致密部.VTA多巴胺能神经元主要投射到伏隔核(nucleus accumbens, NAc),内侧前额叶皮层(medial prefrontal cortex, mPFC)和基底外侧杏仁核(basolateral amygdala, BLA).这些通路是正性奖赏环路的重要组成部分.此外, 基底外侧杏仁核的谷氨酸能神经元不能直接投射到VTA, 但它可以通过伏隔核(Johnson, Aylward, Hussain, & Totterdell, 1994)实现间接投射.内侧前额叶皮层对VTA和伏隔核都有投射(Juarez & Han, 2016), 它们共同构成了正性奖赏环路(图1).其中, VTA富含多巴胺能神经元, 是调控奖赏功能的主要区域(Bowers, Chen, & Bonci, 2010).除了自然奖赏外, 它在药物成瘾诱导的奖赏效应中也发挥重要作用.几乎所有成瘾药物(如尼古丁,酒精类,阿片类和大麻类等)都会直接或间接作用于该区域的多巴胺能神经元(Ikemoto & Bonci, 2014), 增加神经元放电和多巴胺的释放量.
VTA多巴胺能神经元编码奖赏的三种不同状态:“符合预期”,“比预期更好”或“比预期更糟”的奖赏(Pignatelli & Bonci, 2015).“比预期更好”或“比预期更糟”的奖赏分别是正预期错误和负预期错误.预期错误通常被大脑用来精炼和优化随后的反应, 并学习新的行为策略以满足需求.VTA多巴胺能神经元表现出两种放电模式:单峰放电和高频放电.预期的奖赏会使这些神经元产生单峰放电, 正预期错误会导致高频放电, 负预期错误则使放电停止(Pignatelli & Bonci, 2015).也就是说, 多巴胺能神经元被奖赏或奖赏预期线索激活, 被奖赏缺失抑制(Hong et al., 2011).而且, 这类神经元的高频放电(即编码正预期错误)在奖赏效应中发挥重要作用.Steinberg等人(2013)通过光遗传学方法证明, 多巴胺能神经元和预期错误之间存在因果关系.此方法采用基因操作技术使特定类型的神经元表达光敏感通道蛋白.这类蛋白在不同波长的光照刺激下选择性地让阳离子或者阴离子通过, 改变膜电位, 进而选择性地兴奋或抑制这类神经元的活性(Miesenböck, 2009).在实验中, 给予奖赏的同时, 用波长为473 nm的蓝光刺激视紫红质通道蛋白2 (channelrhodopsin-2, ChR2)激活多巴胺能神经元模拟正预期错误, 足以使线索诱导的奖赏-寻求行为持续增加.实际上, 大部分多巴胺能神经元编码的信号与高频放电产生的正预期错误一致.正预期错误的编码可以作为“教”的信号, 并带来正强化(Fields & Margolis, 2015).这些神经元放电还足以在没有外部线索诱导的情况下重新激活先前消退的觅食行为(Adamantidis et al., 2011).由此可知, VTA多巴胺能神经元在奖赏编码,学习和动机中扮演重要角色.
VTA多巴胺能神经元投射到伏隔核, 用于编码奖赏效价和动机凸显(Fields & Margolis, 2015); 投射到内侧前额叶皮层, 主要是调节执行控制; 投射到基底外侧杏仁核, 提高对奖赏背景的联想学习(Russo & Nestler, 2013).研究发现, 高频放电的多巴胺能神经元位于VTA内侧后部, 并投射到内侧前额叶皮层,伏隔核核部或伏隔核内侧壳部.相反, 单峰放电的多巴胺能神经元位于VTA外侧和黑质致密部, 并投射到伏隔核外侧壳部和背侧纹状体(Lammel et al., 2008).这表明不同放电模式的多巴胺能神经元位于VTA的不同区域, 然后投射到不同的脑区发挥作用.此外, 不同类型的刺激也会出现类似的效应.奖赏刺激选择性地影响那些投射到伏隔核内侧壳部的多巴胺能神经元, 而厌恶刺激选择性地影响那些投射到内侧前额叶皮层的多巴胺能神经元.相反, 奖赏和厌恶两种刺激都能影响那些投射到伏隔核外侧壳部的多巴胺能神经元活性(Lammel, Ion, Roeper, & Malenka, 2011).对此可能的解释是, 多巴胺系统的每个奖赏通路受到不同的动机相关刺激的影响.
2.1.2 负性奖赏环路
外侧缰核(lateral habenula, LHb)被认为是负性奖赏环路的中心, 在奖赏环路的负反馈中发挥重要作用(Hong & Hikosaka, 2008; Matsumoto & Hikosaka, 2007).LHb接收来自苍白球(globus pallidus),下丘脑和前扣带回等脑区的输入, 然后直接和间接投射到VTA的多巴胺能神经元(Petzel, Bernard, Poller, & Veh, 2017).LHb和VTA的投射是双向的, 即VTA也可以投射到LHb抑制其神
经元活性(Stamatakis et al., 2013).这些通路共同构成了负性奖赏环路(图1).LHb和中脑多巴胺能神经元在奖赏调控中发挥相反的作用.LHb主要编码负预期错误, 被非奖赏预期目标激活, 被奖赏预期目标抑制(Hong & Hikosaka, 2008).使用fMRI研究人类奖赏相关脑区的激活情况也发现, 当出现负预期错误时缰核处于激活状态(Salas, Baldwin, de Biasi, & Montague, 2010).进一步的研究还显示, LHb也会被厌恶刺激和意外的奖赏缺失所激活(Matsumoto & Hikosaka, 2009), 参与厌恶信息的加工, 调节厌恶刺激诱导的行为反应.
来自LHb的输入可以抑制多巴胺系统.用单脉冲刺激(0.5 mA, 200 ms)大鼠的LHb, 可以短暂抑制97%的VTA/黑质致密部多巴胺能神经元的活性(Ji & Shepard, 2007).同样的发现也出现在用单个双脉冲刺激(100 mA, 0.2 ms)猴子LHb的实验中:在电刺激后的10至40 ms, 该脑区82%的多巴胺能神经元被显著抑制(Matsumoto & Hikosaka, 2007).虽然电刺激的强度和持续时间不尽相同, 但结果都表现出强烈的抑制作用.这可能是LHb和多巴胺系统对奖赏和非奖赏刺激作出相反反应的原因.简而言之, LHb通过抑制多巴胺能神经元, 进而实现负性奖赏调控.因此, LHb-VTA通路被认为是负性奖赏环路的重要组成部分(Hong et al., 2011).然而, LHb富含兴奋性的谷氨酸能神经元, 不可能直接抑制多巴胺能神经元, 这说明此作用需要一个介于LHb和VTA之间的抑制性转换器(Lavezzi & Zahm, 2011)来实现.先前研究发现, 中脑多巴胺能神经元会被GABA能神经元抑制(Ji & Shepard, 2007; Steffensen, Svingos, Pickel, & Henriksen, 1998).单独激活GABAA受体可以抑制多巴胺能神经元的爆发性放电, 而阻断该受体则促进神经元放电(Lobb, Wilson, & Paladini, 2010; Paladini, Celada, & Tepper, 1999).所以, LHb的谷氨酸能神经元被认为是通过GABA能神经元的转换来调控VTA的多巴胺能神经元.近年来发现, GABA能神经元多积聚在RMTg上(Kaufling & Aston-Jones, 2015).因此, RMTg成为这一中间转换器的最佳候选者, 受到许多研究者的关注.
2.2 RMTg是奖赏环路的中间转换器
RMTg接受来自LHb的密集输入.LHb的轴突(>55%)末端多与RMTg GABA能神经元直接发生联系(Balcita-Pedicino, Omelchenko, Bell, & Sesack, 2015).RMTg接受到来自LHb的刺激后, 大约80%的GABA能神经元表达c-Fos (Lammel et al., 2012).足底电击及其相关的条件性刺激也会诱导这两个脑区表达c-Fos.c-Fos的表达有助于编码厌恶信息(Jhou, Fields, Baxter, Saper, & Holland, 2009).不过, 损伤缰核脚间束后, 对这一效应的抑制只在使用低强度的电击中被发现, 即LHb只调节RMTg GABA能神经元对轻度厌恶刺激的反应(Hong et al., 2011).进一步的研究发现, 将小鼠暴露在厌恶刺激下, LHb的兴奋性冲动会传递到RMTg.使用光遗传学方法激活这一通路, 则在RMTg GABA能神经元中产生兴奋性突触后电流, 为RMTg和VTA提供厌恶信号(Jennings et al., 2013; Jhou et al., 2013).这也会诱导强烈的条件性位置厌恶(Lammel et al., 2012),增加回避行为以及抑制正强化,促进负强化(Stamatakis & Stuber, 2012).通过损伤LHb或RMTg,或者用光遗传学方法选择性失活RMTg, 都可以消除回避行为(Jhou et al., 2013).这些研究表明, LHb-RMTg通路主要用于传递负性奖赏信号并增加回避行为.
RMTg密集投射到VTA并抑制多巴胺能神经元的活性.它的大多数神经元轴突优先作用于VTA多巴胺能神经元的树突(Balcita-Pedicino et al., 2015).通过RMTg注射顺行示踪剂或VTA注射逆行示踪剂来探究RMTg-VTA通路, 在VTA轴突中检测到顺行示踪剂, 在76%的RMTg胞体中检测到逆行示踪剂(Jalabert et al., 2011).其他研究同样也发现, VTA多巴胺能神经元主要接收来自RMTg GABA能神经元的输入(Matsui, Jarvie, Robinson, Hentges, & Williams, 2014; Matsui & Williams, 2011).另外, RMTg的GABA能神经元对多巴胺能神经元起抑制作用.使用电生理学(Jalabert et al., 2011; Lecca, Melis, Luchicchi, Muntoni, & Pistis, 2012)和光遗传学方法(Matsui et al., 2014)激活RMTg, 均能强烈抑制VTA多巴胺能神经元的放电频率.损伤大鼠的RMTg后, 这种抑制作用消失, VTA多巴胺能神经元的放电增加, 大鼠的自发活动也增多(Brown et al., 2017).由此推测RMTg的抑制机制可能是:刺激RMTg使GABA能神经元末端释放GABA, 与VTA多巴胺能神经元上的对应受体结合, 打开离子通道, 导致突触后电位超极化.
RMTg是LHb和VTA之间的转换器.它把来自LHb谷氨酸能的兴奋性信号转化为GABA能的抑制性信号(Jhou, Geisler, et al., 2009), 进而抑制VTA多巴胺能神经元的活性.对啮齿类动物的研究发现, 刺激LHb可以激活RMTg GABA能神经元, 使投射到伏隔核壳部的多巴胺能神经元产生抑制性突触后电流(Lammel et al., 2012).在施加厌恶刺激(重复足底电击,预期电击线索,食物剥夺或奖赏缺失)后, RMTg表现出激活; 在出现奖赏或奖赏预期线索后, RMTg受到抑制(Jhou, Fields, et al., 2009; Sánchez-Catalán et al., 2017).对灵长类动物的研究也发现, RMTg接受来自LHb的兴奋输入, 再投射到轴突末端的多巴胺能神经元.RMTg被非奖赏预期目标激活, 被奖赏预期目标抑制, 这种负性奖赏调节与LHb类似, 但与VTA多巴胺能神经元相反(Hong et al., 2011).这些结果表明, RMTg将LHb编码的负预期错误信号传递到VTA, 抑制多巴胺系统的奖赏效应.此外, Brown等人(2017)为RMTg是中间转换器这一观点提供了直接的证据支持.神经兴奋性毒素喹啉酸(Quinolinic acid)可以显著减少RMTg中阳性细胞的数量, 同时又可保留邻近区域的细胞.用其损伤RMTg后再激活LHb, 结果发现受抑制的多巴胺能神经元减少, 同时抑制持续时间缩短, 多巴胺能神经元的放电增加.在旷场实验中, RMTg受损大鼠的自发活动也增加.
综上所述, RMTg主要参与负预期错误,厌恶信息加工以及运动控制.来自广泛大脑区域(最主要的是LHb)的厌恶刺激信息汇聚到RMTg, 再传递至VTA, 使多巴胺系统表现出相反的奖赏效应和行为活动.把这些结构作为一个通路来考虑, RMTg被认为是汇聚和整合多通道信号到多巴胺系统的一个枢纽(Bourdy & Barrot, 2012), 是奖赏系统的一个综合调节器(Brown et al., 2017; Lavezzi & Zahm, 2011).来自LHb的负性奖赏信号通过RMTg传递到多巴胺系统, 抑制正性奖赏环路(Hong et al., 2011), 构成一条关键的负性奖赏调控通路(见图1).
图1
图1
RMTg是奖赏环路和阿片成瘾的中间调节器(参考Juarez & Han, 2016)
LHb, 外侧缰核; RMTg, 喙内侧被盖核; VTA, 腹侧被盖区; mPFC, 内侧前额叶皮层; NAc, 伏隔核; BLA, 基底外侧杏仁核; Glu, 谷氨酸; GABA, γ-氨基丁酸; DA, 多巴胺.
3 阿片类物质作用于RMTg影响奖赏环路
奖赏环路功能异常是药物成瘾最重要的神经机制.研究者们认为, 药物成瘾可能是通过抑制负性奖赏环路和激活正性奖赏环路而导致的(Lecca et al., 2011).药物成瘾的一个核心特征是明知有负面和不愉快的后果仍持续觅药和用药, 这可能是由于调节厌恶的负性奖赏环路受到抑制.多年来对阿片类物质成瘾机制的研究为这些观点提供了大量的证据支持.
阿片类物质的止痛和奖赏效应均与μ-阿片受体有关.μ-阿片受体在树突和突触前表达, 通过打开膜上的钾离子通道导致超极化或者通过减少突触前囊泡释放相应的神经递质, 抑制神经元活性(Fields & Margolis, 2015).早期研究者们认为, 阿片成瘾的机制是, 这类物质直接激活中脑边缘多巴胺系统.但是, Gysling和Wang (1983)发现, 给药后VTA中的非多巴胺能神经元受到抑制.据此猜测, 阿片类物质可能是通过抑制这些神经元间接增加多巴胺能神经元的活性.随后的研究证明, 阿片类物质激活μ-阿片受体后会抑制GABA能神经元, 使VTA多巴胺能神经元去抑制, 从而增加多巴胺的释放(Johnson & North, 1992).此后, 这一通路一致被认为是阿片成瘾的关键机制.投射到VTA多巴胺能神经元上的GABA抑制性突触后电流主要通过三条路径产生:VTA中间神经元,伏隔核和RMTg.在离体实验中, 选择性激活这三个脑区中的GABA能神经元即可产生抑制性突触后电流.然后, 使用剂量为1 μmol的吗啡进行抑制.结果检测到RMTg中产生的抑制性突触后电流减少了46%, 伏隔核中减少了18%, VTA中间神经元产生的此类电流几乎未受影响(Matsui et al., 2014).使用μ-阿片受体激动剂脑啡肽(DAMGO)也检测到类似的抑制作用, 并且随着脑啡肽剂量的增加, 抑制作用逐渐增强.由于阿片类物质对RMTg的抑制作用是三个中最为重要的.所以, 该脑区在阿片成瘾中的作用, 近年来受到广泛关注.
RMTg中富含GABA能神经元, μ-阿片受体在这些神经元上高度表达(Jhou, Geisler, et al., 2009; Sanchez-Catalan et al., 2014; Wasserman, Tan, Kim, & Yeomans, 2016).通过外周注射和RMTg定位注射阿片类物质后, RMTg GABA能神经元的放电频率显著降低(Lecca et al., 2012; Matsui & Williams, 2011), 而VTA多巴胺能神经元的放电频率增加(Lecca et al., 2011).阿片类物质对RMTg的这种抑制作用与GABAA受体激动剂(如蝇蕈醇)的效果类似.向RMTg中注射蝇蕈醇, 其与GABAA受体结合后打开氯离子通道, 实现超极化, 选择性地抑制GABA能神经元.结果发现, VTA多巴胺能神经元的放电频率和爆发率都增加, 还可以阻断注射到VTA的吗啡对多巴胺能神经元的激活作用(Jalabert et al., 2011).这说明阿片类物质主要作用于RMTg上的μ-阿片受体, 两者结合后可能打开GABAA控制的氯离子通道, 进而抑制GABA能神经元.此外, RMTg GABA能神经元也会被μ-阿片受体激动剂抑制.例如, 给予μ-阿片受体激动剂脑啡肽后, RMTg GABA能神经元的自发放电大幅减少, 并且膜电位超极化.然而, 使用κ-和δ-阿片受体激动剂并未发挥这一作用.采用电刺激和光遗传学方法激活RMTg, 可以诱发多巴胺能神经元上GABAA的抑制性突触后电流, 同样这一作用只被μ-阿片受体激动剂所抑制(Matsui & Williams, 2011).这些结果证明, 阿片类物质作用于μ-阿片受体, 抑制RMTg GABA能神经元, 进而实现对VTA多巴胺能神经元的激活作用.因此, RMTg是阿片类物质的重要作用靶点, RMTg到VTA的功能联结是中脑奖赏系统和阿片成瘾的关键通路.
行为研究发现, RMTg调节阿片类物质在奖赏行为和自发活动中的作用.自发活动是接近行为的关键成分.奖赏和接近行为处于稳态的协调加工过程中.正性奖赏操作可以促进接近行为, 而负性奖赏操作则抑制正在进行的接近行为, 并诱导厌恶情绪(Ikemoto & Bonci, 2014).由此可知, 激活正性奖赏环路会诱导自发活动, 反之会抑制自发活动.研究发现, 与其他脑区相比, 内吗啡肽1 (endomorphin-1)通过自身给药方式注入RMTg导致的自身给药率最高; 同时, 内吗啡肽1注入RMTg而非其他脑区产生了条件性位置偏爱.这一效应与RMTg注射GABAA受体激动剂蝇蕈醇的效应类似(Jhou, Fields, et al., 2009).VTA后侧注射吗啡延长了两次海洛因自身给药的时间间隔.其中, 把吗啡注射到RMTg时, 两次自身给药的间隔时间最长.这说明吗啡作用于RMTg时, 大鼠获得了最强的满足感, 这一脑区是吗啡强化工具性行为(instrumental behavior)最有效的部位(Steidl, Myal, & Wise, 2015).此外, RMTg注入吗啡(Steidl, Dhillon, Sharma, & Ludwig, 2017)或者μ-阿片受体激动剂脑啡肽(Kotecki et al., 2015), 啮齿类动物的自发活动也显著增加.Kotecki等人(2015)给小鼠的VTA和RMTg注射四种剂量(0.01 nmol,0.1 nmol,1 nmol和10 nmol)的脑啡肽.随着剂量的增加, 自发活动呈先上升后下降的趋势.其中注射到VTA的脑啡肽剂量为1 nmol时自发活动达到最大值, 而注射到RMTg的剂量仅为0.1 nmol时即可达到最大值.这说明RMTg对阿片类物质的反应更敏感.
总的来说, 阿片类物质主要通过抑制RMTg GABA能神经元, 使VTA多巴胺能神经元去抑制, 进而激活正性奖赏环路(图1).这一作用诱导的行为表现是自身给药,条件性位置偏爱和自发活动显著增加, 最终导致阿片类物质成瘾.简而言之, RMTg是奖赏环路和阿片成瘾的中间调节器.
4 RMTg的GABA能神经元和胆碱能受体在阿片成瘾中的作用
阿片成瘾的主要机制是阿片类物质作用于RMTg的μ-阿片受体, 抑制GABA能神经元活性, 进而激活中脑边缘多巴胺系统.目前已有研究者开始根据这一机制寻求治疗阿片成瘾的新方法, 关注的靶点主要集中在RMTg的GABA能神经元和毒蕈碱型胆碱能受体(M胆碱受体).
4.1 RMTg的GABA能神经元在阿片类物质戒断中的作用
近期的研究探讨了RMTg在阿片类物质戒断中的贡献, 发现急性和慢性吗啡戒断对RMTg GABA能神经元的去抑制作用有不同影响.
急性戒断导致吗啡对RMTg GABA能神经元的抑制作用失效.Sánchez-Catalán等人(2017)发现, 纳洛酮催促吗啡戒断会诱导RMTg的μ-阿片受体阳性(15%)和阴性(85%)细胞表达c-Fos.这说明戒断期间, 在RMTg中可能存在直接和间接机制.阳性细胞的表达被认为是直接诱导的, 阴性细胞的表达可能是通过RMTg输入和多突触环路间接诱导的.再者, RMTg GABA能神经元的放电频率(Kaufling & Aston-Jones, 2015)和GABAA 抑制性突触后电流(Matsui et al., 2014)在纳洛酮催促戒断的大鼠和正常大鼠之间无显著差异.也就是说, 纳洛酮戒断使吗啡对这些神经元的抑制作用减弱, 这些神经元趋于正常化, 即恢复对多巴胺能神经元的抑制能力.
慢性吗啡戒断后RMTg GABA能神经元仍受抑制.Kaufling和Aston-Jones (2015)发现, 慢性吗啡戒断(2周)大鼠的这些神经元的放电频率与吗啡依赖大鼠类似, 都显著低于正常大鼠.通过光遗传学方法激活RMTg, 发现其抑制了正常大鼠和慢性戒断大鼠的VTA多巴胺能神经元.然而, 通过注射GABAA受体激动剂(蝇蕈醇)抑制RMTg GABA能神经元, 却只增加了正常大鼠而非慢性戒断大鼠的多巴胺能神经元的活性.这说明RMTg对多巴胺能神经元的抑制能力在持续戒断期间仍被保留, 但是去抑制能力受损.换而言之, 戒断状态下RMTg会持续抑制多巴胺能神经元的活性.厌恶信号传入会激活RMTg抑制多巴胺系统; 但奖赏信号传入却不能消除RMTg对多巴胺能神经元的去抑制作用, 无法激活多巴胺系统.其结果导致慢性戒断大鼠维持长久的负性情绪状态.这可能是成瘾药物戒断后出现负性情绪体验的主要原因.
关于RMTg对吗啡耐受性作用的研究却得出了不一致的结论.慢性吗啡戒断1周, 随后用纳洛酮进行急性戒断, RMTg对吗啡的抑制作用表现出局部耐受性(Matsui et al., 2014).然而, Kaufling和Aston-Jones (2015)却发现, 给正常组,吗啡依赖组和吗啡戒断组(戒断2周)急性注射吗啡和选择性μ-阿片受体激动剂脑啡肽, 三组动物的RMTg放电频率均出现了相当程度地降低.这说明三个组的RMTg GABA能神经元受到吗啡的抑制效应无差异, 即无耐受性(Kaufling & Aston- Jones, 2015).不一致的结果可能是由于两者的测量方法不同, 前者是离体测量, 而后者是在体测量.至于RMTg对吗啡是否有耐受性还需要更多的研究来证实.
4.2 RMTg的M胆碱受体在阿片成瘾中的作用
先前的研究发现, VTA中的胆碱能信号会影响阿片类物质诱导的奖赏效应.例如, VTA定位注射M胆碱受体拮抗剂, 东莨菪碱完全阻断了吗啡诱导的多巴胺的释放(Steidl, Miller, Blaha, & Yeomans, 2011), 阿托品阻断了吗啡诱导的条件性位置偏爱(Rezayof, Nazari-Serenjeh, Zarrindast, Sepehri, & Delphi, 2007).那么, RMTg中的胆碱能系统是否会出现类似或相反的作用呢?近年来, 有学者对此研究发现, 胆碱能在VTA和RMTg中作用相反.将吗啡和阿托品联合注射到VTA中, 完全抑制了吗啡诱导的自发活动; 但注射到RMTg, 自发活动却显著增加(Steidl, Dhillon, et al., 2017).换而言之, 在阿片类物质诱导的奖赏效应中, VTA的胆碱能系统起激活作用, 而RMTg的胆碱能系统起抑制作用.这有可能成为治疗阿片成瘾的新思路.
另有实验考察了M胆碱受体的不同亚型如何影响吗啡对RMTg的抑制作用.运用化学基因法将非内源性M5受体基因转录到小鼠的VTA或RMTg神经元中, 结果发现, 在外周注射两种吗啡剂量(10和30 mg/kg)下, M5转录到VTA的小鼠自发活动都增加了一倍多, M5转录到RMTg的小鼠自发活动被显著抑制, 减少了64%到83%.这说明相同的M5基因转录到RMTg中产生了显著的抑制行为效应, 与其在VTA中的激活效应相反(Wasserman, Wang, Rashid, Josselyn, & Yeomans, 2013).同样的方法又用于研究M3和M4受体在RMTg中的表达.结果显示, M3和M4受体都在μ-阿片受体标记的神经元附近被发现, M4受体的比例高于M3受体.用N-氧化氯氮平(clozapine-N- oxide)激活在RMTg中表达的两种受体会产生完全相反的效应.激活M3受体会增强RMTg GABA能神经元的活性, 减少外周注射吗啡诱导的自发活动; 而激活M4受体则会抑制这类神经元的活性, 增加自发活动(Wasserman et al., 2016).其他研究进一步探讨M胆碱受体如何影响RMTg定位注射吗啡的作用.RMTg联合注射吗啡和M3-选择性拮抗剂4-DAMP, 结果吗啡诱导的自发活动显著增加.不过, 联合注射吗啡和M4-选择性拮抗剂托吡卡胺(Tropicamide), 自发活动并未改变(Steidl, Dhillon, et al., 2017).这些结果表明, RMTg中M胆碱受体的不同亚型在阿片类物质激活的奖赏效应中发挥不同作用.激活M3和M5受体可能起抑制作用, 而激活M4受体可能发挥相反作用或者未发挥作用.
以上研究表明, RMTg的M胆碱受体在阿片类物质诱导的奖赏效应中起重要作用, 有可能成为治疗阿片成瘾的一个重要靶点.然而, 由于实验方法和给药方式不同, 这些研究结果不能直接进行比较.目前, 无论是M胆碱受体拮抗剂在外周注射吗啡诱导的自发活动中的作用, 还是M胆碱受体亚型的表达在RMTg注射吗啡诱发的自发活动中的作用都未被探测过(Steidl, Wasserman, Blaha, & Yeomans, 2017).今后需要对此进行深入的研究和比较.
5 不足与展望
RMTg是LHb和中脑多巴胺系统的转换器, 又是阿片类物质的重要作用靶点, 所以它在奖赏环路和阿片成瘾中起关键作用, 是两者的中间调节器.深入探讨这一区域, 有助于揭示阿片成瘾的神经机制.虽然近年来对该领域的研究已经取得不少成果, 但是仍有一些问题需要关注和解决.
首先, 目前大多数的研究聚焦于正性奖赏环路(尤其是VTA-伏隔核通路), 而对负性奖赏环路的研究仍不充分.LHb-RMTg-VTA负性奖赏通路可以抑制中脑多巴胺能神经元的活性, 降低奖赏效应.所以, 刺激LHb-RMTg这一通路极有可能解除阿片类物质对RMTg GABA能神经元的抑制作用, 即抑制阿片奖赏效应, 最终达到治疗效果.采用深部脑刺激(deep brain stimulation)技术的动物研究已经验证, 高低频交替刺激LHb可以减弱大鼠的可卡因觅药行为(Friedman et al., 2010).今后的研究可以使用此项技术直接激活RMTg或LHb-RMTg通路, 深入考察其对阿片成瘾的抑制作用.此外, 这项技术是否适合作为人类药物成瘾的治疗手段, 值得进一步探讨.
其次, 关于RMTg调控中脑边缘多巴胺系统的研究多集中在啮齿类和灵长类动物, 是否同样的效应也出现在人类中还有待证实.此外, RMTg是加工厌恶信息和诱导回避行为的主要脑区, 但并非唯一脑区.所以, 深入探究相关通路, 更为全面地了解RMTg在调节奖赏和厌恶神经环路中发挥的作用是必要的.还有研究发现激活RMTg可以促进觅药行为的消退.Huff和LaLumiere (2015)在消退训练前或训练后, 向大鼠的RMTg中注射谷氨酸AMPA受体正向变构调节剂PEPA, 发现促进了消退学习的保持.之所以选择PEPA是因为它本身不会直接激活谷氨酸受体, 而且它还只允许内源性的谷氨酸激活受体.在消退训练之后失活RMTg, 大鼠自身给药的压杆量增加.这说明RMTg可能是弱化觅药动机和促进消退的重要脑区.未来需要深入探讨这一作用, 有助于我们了解成瘾药物戒断和复吸的神经机制.
最后, 关于RMTg在阿片类物质戒断中的作用的研究还较少, 结果多是通过电生理学和光遗传学方法测得.虽然发现纳洛酮催促吗啡戒断使RMTg GABA能神经元恢复对多巴胺系统的抑制作用, 但对戒断后的情绪和行为还未进行具体研究.慢性戒断并没有恢复这些神经元的活性, 戒断后的负性情绪可能与RMTg密切相关, 未来的研究可以关注这一方向.近年来的研究发现, 胆碱类物质作用于RMTg中的M胆碱受体可以抑制吗啡诱导的自发活动(Wasserman et al., 2016; Wasserman et al., 2013), 这有可能成为治疗阿片成瘾的突破口.然而, M胆碱受体与RMTg GABA能神经元的具体作用机制尚不清楚.胆碱类物质对RMTg GABA能神经元的激活作用为什么强于阿片类物质对它们的抑制作用?RMTg中是否还存在其他M胆碱受体亚型(例如M1或M2)?这些问题目前仍无法解答, 所以今后还需要进一步探讨.
参考文献
Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior.
DOI:10.1523/JNEUROSCI.2246-11.2011
URL
PMID:3171183
[本文引用: 1]

Abstract Phasic activation of dopaminergic neurons is associated with reward-predicting cues and supports learning during behavioral adaptation. While noncontingent activation of dopaminergic neurons in the ventral tegmental are (VTA) is sufficient for passive behavioral conditioning, it remains unknown whether the phasic dopaminergic signal is truly reinforcing. In this study, we first targeted the expression of channelrhodopsin-2 to dopaminergic neurons of the VTA and optimized optogenetically evoked dopamine transients. Second, we showed that phasic activation of dopaminergic neurons in freely moving mice causally enhances positive reinforcing actions in a food-seeking operant task. Interestingly, such effect was not found in the absence of food reward. We further found that phasic activation of dopaminergic neurons is sufficient to reactivate previously extinguished food-seeking behavior in the absence of external cues. This was also confirmed using a single-session reversal paradigm. Collectively, these data suggest that activation of dopaminergic neurons facilitates the development of positive reinforcement during reward-seeking and behavioral flexibility.
The inhibitory influence of the lateral habenula on midbrain dopamine cells: Ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus.
A new control center for dopaminergic systems: Pulling the VTA by the tail.
DOI:10.1016/j.tins.2012.06.007
URL
PMID:22824232
Magsci
[本文引用: 1]

The tail of the ventral tegmental area (tVTA), also named the rostromedial tegmental nucleus (RMTg), is a recently defined midbrain structure considered to exert a major inhibitory drive on dopamine systems. In view of its connectivity, tVTA is well placed to convey salient positive and negative signals to dopamine cells and participate in adaptative behavioral responses. This structure could act as a hub converging and integrating widespread multimodal signals toward dopamine systems. The tVTA participates in prediction error, motor control, and responses to aversive stimuli and drugs of abuse. In light of the crucial role of the tVTA in the opiate control of dopamine activity, a neuroanatomical update of the disinhibition model of morphine action is proposed.
AMPA receptor synaptic plasticity induced by psychostimulants: The past, present, and therapeutic future.
DOI:10.1016/j.neuron.2010.06.004
URL
PMID:2904302
Magsci
[本文引用: 1]

Experience-dependent plasticity at excitatory synapses of the mesocorticolimbic system is a fundamental brain mechanism that enables adaptation to an ever-changing environment. These synaptic responses are critical for the planning and execution of adaptive behaviors that maximize survival. The mesocorticolimbic system mediates procurement of positive reinforcers such as food and sex; however, drugs of abuse resculpt this crucial circuitry to promote compulsive drug-seeking behavior. This review will discuss the long-term changes in glutamatergic neurotransmission that occur within the mesolimbic system following cocaine exposure. In addition, we will examine how these long-lasting neuroadaptations may drive the pathology of psychostimulant addiction. Finally, we review clinical trials that highlight antagonists at excitatory AMPA receptors as promising targets against cocaine abuse.
Habenula-induced inhibition of midbrain dopamine neurons is diminished by lesions of the rostromedial tegmental nucleus.
DOI:10.1523/JNEUROSCI.1353-16.2017
URL
PMID:5214632
[本文引用: 2]

Neurons in the lateral habenula (LHb) are transiently activated by aversive events and have been implicated in associative learning. Functional changes associated with tonic and phasic activation of the LHb are often attributed to a corresponding inhibition of midbrain dopamine (DA) neurons. Activation of GABAergic neurons in the rostromedial tegmental nucleus (RMTg), a region that receives dense projections from the LHb and projects strongly to midbrain monoaminergic nuclei, is believed to underlie the transient inhibition of DA neurons attributed to activation of the LHb. To test this premise, the effects of axon-sparing lesions of the RMTg were assessed on LHb-induced inhibition of midbrain DA cell firing in anesthetized rats. Quinolinic acid lesions decreased the number of NeuN-positive neurons in the RMTg significantly while largely sparing cells in neighboring regions. Lesions of the RMTg reduced both the number of DA neurons inhibited by, and the duration of inhibition resulting from, LHb stimulation. Although the firing rate was not altered, the regularity of DA cell firing was increased in RMTg-lesioned rats. Locomotor activity in an open field was also elevated. These results are the first to show that RMTg neurons contribute directly to LHb-induced inhibition of DA cell activity and support the widely held proposition that GABAergic neurons in the mesopontine tegmentum are an important component of a pathway that enables midbrain DA neurons to encode the negative valence associated with failed expectations and aversive stimuli.SIGNIFICANCE STATEMENT Phasic changes in the activity of midbrain dopamine cells motivate and guide future behavior. Activation of the lateral habenula by aversive events inhibits dopamine neurons transiently, providing a neurobiological representation of learning models that incorporate negative reward prediction errors. Anatomical evidence suggests that this inhibition occurs via the rostromedial tegmental nucleus, but this hypothesis has yet to be tested directly. Here, we show that axon-sparing lesions of the rostromedial tegmentum attenuate habenula-induced inhibition of dopamine neurons significantly. These data support a substantial role for the rostromedial tegmentum in habenula-induced feedforward inhibition of dopamine neurons.
Understanding opioid reward.
Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior.
DOI:10.1016/j.neuropharm.2010.06.008
URL
PMID:20600170
Magsci
[本文引用: 2]

The lateral habenula (LHb) is critical for modulation of negative reinforcement, punishment and aversive responses. In light of the success of deep-brain-stimulation (DBS) in the treatment of neurological disorders, we explored the use of LHb DBS as a method of intervention in cocaine self-administration, extinction, and reinstatement in rats. An electrode was implanted into the LHb and rats were trained to self-administer cocaine (21 days; 0.25–102mg/kg) until they achieved at least three days of stable performance (as measured by daily recordings of active lever presses in self-administration cages). Thereafter, rats received DBS in the presence or absence of cocaine. DBS reduced cocaine seeking behavior during both self-administration and extinction training. DBS also attenuated the rats’ lever presses following cocaine reinstatement (5–2002mg/kg) in comparison to sham-operated rats. These results were also controlled by the assessment of physical performance as measured by water self-administration and an open field test, and by evaluation of depressive-like manifestations as measured by the swim and two-bottles-choice tests. In contrast, LHb lesioned rats demonstrated increased cocaine seeking behavior as demonstrated by a delayed extinction response. In the ventral tegmental area, cocaine self-administration elevated glutamatergic receptor subunits NR1 and GluR1 and scaffolding protein PSD95, but not GABA Aβ, protein levels. Following DBS treatment, levels of these subunits returned to control values. We postulate that the effect of both LHb modulation and LHb DBS on cocaine reinforcement may be via attenuation of the cocaine-induced increase in glutaminergic input to the VTA.
Morphine-induced activation of A10 dopamine neurons in the rat.
DOI:10.1016/0006-8993(83)90913-7
URL
PMID:6315137

The effects of intravenous administration of morphine (MOR) on the spontaneous discharge rate of dopamine (DA) neurons in the ventral tegmental area (VTA or A10) and the substantia nigra pars compacta (SNC or A9) were compared. MOR (0.5-3.5 mg/kg) produced a marked increase in the spontaneous firing of both A10 and A9 DA neurons. Naloxone (NAL) reversed the MOR effects. Acute transection of the medial forebrain bundle (MFB) did not interfere with the observed MOR effects on either A10 or A9 DA neurons. However, following chronic lesions of the MFB (6 days), A9 DA neurons were no longer responsive to MOR whereas A10 DA cells were still activated by MOR. Neither radiofrequency lesions of the dorsal raphe nucleus (DNR) nor administration of the 5-HT 2 antagonist ketanserin affected the stimulatory effect of MOR on either A10 or A9 DA cells. Thus, it is confirmed that the effects of MOR on A9 DA cells depend on striatonigral feedback pathways. In contrast, it appears that the MOR-induced activation of A10 DA cells does not depend on afferents from the forebrain or on projections from the DRN, suggesting a more direct action of MOR on A10 DA cells. Microiontophoretic application of MOR or enkephalin analogues significantly increased the spontaneous activity of both A9 and A10 DA cells. However, these effects were not reversed by either iontophoretic or intravenous NAL. On the other hand, both intravenously (0.5-1.5 mg/kg) and iontophoretically administered MOR markedly suppressed the electrical activity of non-DA cells found in the vicinity of A10 DA neurons, and this effect was completely reversed by NAL. It is proposed that the MOR-induced activation of A10 DA cells could be mediated indirectly by non-DA cells.
The reward circuit: Linking primate anatomy and human imaging.
DOI:10.1038/npp.2009.129
URL
PMID:19812543
[本文引用: 2]

Abstract Although cells in many brain regions respond to reward, the cortical-basal ganglia circuit is at the heart of the reward system. The key structures in this network are the anterior cingulate cortex, the orbital prefrontal cortex, the ventral striatum, the ventral pallidum, and the midbrain dopamine neurons. In addition, other structures, including the dorsal prefrontal cortex, amygdala, hippocampus, thalamus, and lateral habenular nucleus, and specific brainstem structures such as the pedunculopontine nucleus, and the raphe nucleus, are key components in regulating the reward circuit. Connectivity between these areas forms a complex neural network that mediates different aspects of reward processing. Advances in neuroimaging techniques allow better spatial and temporal resolution. These studies now demonstrate that human functional and structural imaging results map increasingly close to primate anatomy.
The globus pallidus sends reward-related signals to the lateral habenula.
DOI:10.1016/j.neuron.2008.09.035
URL
PMID:19038227
Magsci
[本文引用: 2]

As a major output station of the basal ganglia, the globus pallidus internal segment (GPi) projects to the thalamus and brainstem nuclei thereby controlling motor behavior. A less well known fact is that the GPi also projects to the lateral habenula (LHb) which is often associated with the limbic system. Using the monkey performing a saccade task with positionally biased reward outcomes, we found that antidromically identified LHb-projecting neurons were distributed mainly in the dorsal and ventral borders of the GPi and that their activity was strongly modulated by expected reward outcomes. A majority of them were excited by the no-reward-predicting target and inhibited by the reward-predicting target. These reward-dependent modulations were similar to those in LHb neurons but started earlier than those in LHb neurons. These results suggest that GPi may initiate reward-related signals through its effects on the LHb, which then influences the dopaminergic and serotonergic systems.
Negative reward signals from lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates.
DOI:10.1523/JNEUROSCI.1384-11.2011
URL
PMID:21832176
[本文引用: 6]

Abstract Lateral habenula (LHb) neurons signal negative "reward-prediction errors" and inhibit midbrain dopamine (DA) neurons. Yet LHb neurons are largely glutamatergic, indicating that this inhibition may occur through an intermediate structure. Recent studies in rats have suggested a candidate for this role, the GABAergic rostromedial tegmental nucleus (RMTg), but this neural pathway has not yet been tested directly. We now show using electrophysiology and anatomic tracing that (1) the monkey has an inhibitory structure similar to the rat RMTg; (2) RMTg neurons receive excitatory input from the LHb, exhibit negative reward-prediction errors, and send axonal projections near DA soma; and (3) stimulating this structure inhibits DA neurons. Surprisingly, some RMTg neurons responded to reward cues earlier than the LHb, and carry "state-value" signals not found in DA neurons. Thus, our data suggest that the RMTg translates LHb reward-prediction errors (negative) into DA reward-prediction errors (positive), while transmitting additional motivational signals to non-DA networks.
The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats.
DOI:10.1038/npp.2014.260
URL
PMID:4330500

Recent findings suggest that the mesolimbic dopamine neurons, known to promote cocaine-seeking behavior, are strongly inhibited by a newly characterized region of the midbrain known as the rostromedial tegmental nucleus (RMTg). The RMTg appears to be involved in generating reward-prediction error signals and inhibition of motivated behaviors, suggesting its potential involvement in the extinction of cocaine seeking as well. Therefore, to address this question, male Sprague-Dawley rats underwent surgeries for implantation of catheters and cannulas targeted at the RMTg. After cocaine self-administration, rats underwent modified extinction training. Pre- or post-training intra-RMTg microinjections of the allosteric AMPA receptor potentiator PEPA during the first 5 days of extinction training appeared to enhance the retention of the extinction learning. Following the extinction training, rats underwent cue-induced reinstatement or an 'inactivation-alone' extinction tests. RMTg inactivation before a cue-induced reinstatement session or inactivation alone before a standard extinction session increased overall lever pressing. To determine whether these effects generalized to other motivated behaviors, additional experiments examining food-seeking behavior were also conducted. The results from the food-seeking experiments indicate that PEPA microinjections into the RMTg did not influence the extinction of food seeking and that, at least in rats that had not been given PEPA during the extinction learning experiments, RMTg inactivation had no effect on lever pressing during the cue-induced reinstatement or inactivation-alone tests. These findings suggest that the RMTg provides general behavioral inhibition and is potentially involved in learning to extinguish cocaine-seeking behavior in rats.
Neurocircuitry of drug reward.
Neuronal circuits underlying acute morphine action on dopamine neurons.
DOI:10.1073/pnas.1105418108
URL
PMID:21930931
Magsci
[本文引用: 3]

National Academy of Sciences
Distinct extended amygdala circuits for divergent motivational states.
DOI:10.1038/nature12041
URL
PMID:3778934
Magsci
[本文引用: 1]

The co-morbidity of anxiety and dysfunctional reward processing in illnesses such as addiction(1) and depression(2) suggests that common neural circuitry contributes to these disparate neuropsychiatric symptoms. The extended amygdala, including the bed nucleus of the stria terminalis (BNST), modulates fear and anxiety(3,4), but also projects to the ventral tegmental area (VTA)(5,6), a region implicated in reward and aversion(7-13), thus providing a candidate neural substrate for integrating diverse emotional states. However, the precise functional connectivity between distinct BNST projection neurons and their postsynaptic targets in the VTA, as well as the role of this circuit in controlling motivational states, have not been described. Here we record and manipulate the activity of genetically and neurochemically identified VTA-projecting BNST neurons in freely behaving mice. Collectively, aversive stimuli exposure produced heterogeneous firing patterns in VTA-projecting BNST neurons. By contrast, in vivo optically identified glutamatergic projection neurons displayed a net enhancement of activity to aversive stimuli, whereas the firing rate of identified GABAergic (gamma-aminobutyric acid-containing) projection neurons was suppressed. Channelrhodopsin-2-assisted circuit mapping revealed that both BNST glutamatergic and GABAergic projections preferentially innervate postsynaptic non-dopaminergic VTA neurons, thus providing a mechanistic framework for in vivo circuit perturbations. In vivo photostimulation of BNST glutamatergic projections resulted in aversive and anxiogenic behavioural phenotypes. Conversely, activation of BNST GABAergic projections produced rewarding and anxiolytic phenotypes, which were also recapitulated by direct inhibition of VTA GABAergic neurons. These data demonstrate that functionally opposing BNST to VTA circuits regulate rewarding and aversive motivational states, and may serve as a crucial circuit node for bidirectionally normalizing maladaptive behaviours.
The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses.
DOI:10.1016/j.neuron.2009.02.001
Magsci
[本文引用: 3]

<h2 class="secHeading" id="section_abstract">Summary</h2><p id="">Separate studies have implicated the lateral habenula (LHb) or amygdala-related regions in processing aversive stimuli, but their relationships to each other and to appetitive motivational systems are poorly understood. We show that neurons in the recently identified GABAergic <em>rostromedial tegmental nucleus</em> (RMTg), which receive a major LHb input, project heavily to midbrain dopamine neurons, and show phasic activations and/or Fos induction after aversive stimuli (footshocks, shock-predictive cues, food deprivation, or reward omission) and inhibitions after rewards or reward-predictive stimuli. RMTg lesions markedly reduce passive fear behaviors (freezing, open-arm avoidance) dependent on the extended amygdala, periaqueductal gray, or septum, all regions that project directly to the RMTg. In contrast, RMTg lesions spare or enhance active fear responses (treading, escape) in these same paradigms. These findings suggest that aversive inputs from widespread brain regions and stimulus modalities converge onto the RMTg, which opposes reward and motor-activating functions of midbrain dopamine neurons.</p>
The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta.
DOI:10.1002/cne.21891
URL
PMID:3116663
[本文引用: 4]

Prior studies revealed that aversive stimuli and psychostimulant drugs elicit Fos expression in neurons clustered above and behind the interpeduncular nucleus that project strongly to the ventral tegmental area (VTA) and substantia nigra (SN) compacta (C). Other reports suggest that these neurons modulate responses to aversive stimuli. We now designate the region containing them as the mesopontine rostromedial tegmental nucleus (RMTg) and report herein on its neuroanatomy. Dense -opioid receptor and somatostatin immunoreactivity characterize the RMTg, as do neurons projecting to the VTA/SNC that are enriched in GAD67 mRNA. Strong inputs to the RMTg arise in the lateral habenula (LHb) and, to a lesser extent, the SN. Other inputs come from the frontal cortex, ventral striatopallidum, extended amygdala, septum, preoptic region, lateral, paraventricular and posterior hypothalamus, zona incerta, periaqueductal gray, intermediate layers of the contralateral superior colliculus, dorsal raphe, mesencephalic, pontine and medullary reticular formation, and the following nuclei: parafascicular, supramammillary, mammillary, ventral lateral geniculate, deep mesencephalic, red, pedunculopontine and laterodorsal tegmental, cuneiform, parabrachial, and deep cerebellar. The RMTg has meager outputs to the forebrain, mainly to the ventral pallidum, preoptic-lateral hypothalamic continuum, and midline-intralaminar thalamus, but much heavier outputs to the brainstem, including, most prominently, the VTA/SNC, as noted above, and to medial tegmentum, pedunculopontine and laterodorsal tegmental nuclei, dorsal raphe, and locus ceruleus and subceruleus. The RMTg may integrate multiple forebrain and brainstem inputs in relation to a dominant LHb input. Its outputs to neuromodulatory projection systems likely converge with direct LHb projections to those structures. J. Comp. Neurol. 513:566-596, 2009. 2009 Wiley-Liss, Inc.
Cocaine drives aversive conditioning via delayed activation of dopamine- responsive habenular and midbrain pathways.
DOI:10.1523/JNEUROSCI.3634-12.2013
URL
PMID:23616555
[本文引用: 2]

Many strong rewards, including abused drugs, also produce aversive effects that are poorly understood. For example, cocaine can produce aversive conditioning after its rewarding effects have dissipated, consistent with opponent process theory, but the neural mechanisms involved are not well known. Using electrophysiological recordings in awake rats, we found that some neurons in the lateral habenula (LHb), where activation produces aversive conditioning, exhibited biphasic responses to single doses of intravenous cocaine, with an initial inhibition followed by delayed excitation paralleling cocaine's shift from rewarding to aversive. Recordings in LHb slice preparations revealed similar cocaine-induced biphasic responses and further demonstrated that biphasic responses were mimicked by dopamine, that the inhibitory phase depended on dopamine D2-like receptors, and that the delayed excitation persisted after drug washout for prolonged durations consistent with findings in vivo . c-Fos experiments further showed that cocaine-activated LHb neurons preferentially projected to and activated neurons in the rostromedial tegmental nucleus (RMTg), a recently identified target of LHb axons that is activated by negative motivational stimuli and inhibits dopamine neurons. Finally, pharmacological excitation of the RMTg produced conditioned place aversion, whereas cocaine-induced avoidance behaviors in a runway operant paradigm were abolished by lesions of LHb efferents, lesions of the RMTg, or by optogenetic inactivation of the RMTg selectively during the period when LHb neurons are activated by cocaine. Together, these results indicate that LHb/RMTg pathways contribute critically to cocaine-induced avoidance behaviors, while also participating in reciprocally inhibitory interactions with dopamine neurons.
Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA (A) receptor-mediated mechanism.
DOI:10.1523/JNEUROSCI.0958-07.2007
URL
PMID:17596440
[本文引用: 2]

Abstract Transient changes in the activity of midbrain dopamine neurons encode an error signal that contributes to associative learning. Although considerable attention has been devoted to the mechanisms contributing to phasic increases in dopamine activity, less is known about the origin of the transient cessation in firing accompanying the unexpected loss of a predicted reward. Recent studies suggesting that the lateral habenula (LHb) may contribute to this type of signaling in humans prompted us to evaluate the effects of LHb stimulation on the activity of dopamine and non-dopamine neurons of the anesthetized rat. Single-pulse stimulation of the LHb (0.5 mA, 100 micros) transiently suppressed the activity of 97% of the dopamine neurons recorded in the substantia nigra and ventral tegmental area. The duration of the cessation averaged approximately 85 ms and did not differ between the two regions. Identical stimuli transiently excited 52% of the non-dopamine neurons in the ventral midbrain. Electrolytic lesions of the fasciculus retroflexus blocked the effects of LHb stimulation on dopamine neurons. Local application of bicuculline but not the SK-channel blocker apamin attenuated the effects of LHb stimulation on dopamine cells, indicating that the response is mediated by GABA(A) receptors. These data suggest that LHb-induced suppression of dopamine cell activity is mediated indirectly by orthodromic activation of putative GABAergic neurons in the ventral midbrain. The habenulomesencephalic pathway, which is capable of transiently suppressing the activity of dopamine neurons at a population level, may represent an important component of the circuitry involved in encoding reward expectancy.
Input from the amygdala to the rat nucleus accumbens: Its relationship with tyrosine hydroxylase immunoreactivity and identified neurons.
DOI:10.1016/0306-4522(94)90408-1
URL
PMID:7530817
[本文引用: 1]

Both tyrosine hydroxylase-positive fibres from the mesolimbic dopamine system and amygdala projection fibres from the basolateral nucleus are known to terminate heavily in the nucleus accumbens. Caudal amygdala fibres travelling dorsally via the stria terminalis project densely to the nucleus accumbens shell, especially in the dopamine rich septal hook. The amygdala has been associated with the recognition of emotionally relevant stimuli while the mesolimbic dopamine system is implicated with reward mechanisms. There is behavioural and electrophysiological evidence that the amygdala input to the nucleus accumbens is modulated by the mesolimbic dopamine input, but it is not known how these pathways interact anatomically within the nucleus accumbens. Using a variety of neuroanatomical techniques including anterograde and retrograde tracing, immunocytochemistry and intracellular filling, we have demonstrated convergence of these inputs on to medium-sized spiny neurons. The terminals of the basolateral amygdala projection make asymmetrical synapses predominantly on the heads of spines which also receive on their necks or adjacent dendrites, symmetrical synaptic input from the mesolimbic dopamine system. Some of these neurons have also been identified as projection neurons, possibly to the ventral pallidum. We have shown a synaptic level how dopamine is positioned to modulate excitatory limbic input in the nucleus accumbens.
Opioids excite dopamine neurons by hyperpolarization of local interneurons.
Diversity of dopaminergic neural circuits in response to drug exposure.
DOI:10.1038/npp.2016.32
URL
PMID:26934955
[本文引用: 3]

Addictive substances are known to increase dopaminergic signaling in the mesocorticolimbic system. The origin of this dopamine (DA) signaling originates in the ventral tegmental area (VTA), which sends afferents to various targets, including the nucleus accumbens, the medial prefrontal cortex, and the basolateral amygdala. VTA DA neurons mediate stimuli saliency and goal-directed behaviors. These neurons undergo robust drug-induced intrinsic and extrinsic synaptic mechanisms following acute and chronic drug exposure, which are part of brain-wide adaptations that ultimately lead to the transition into a drug-dependent state. Interestingly, recent investigations of the differential subpopulations of VTA DA neurons have revealed projection-specific functional roles in mediating reward, aversion, and stress. It is now critical to view drug-induced neuroadaptations from a circuit-level perspective to gain insight into how differential dopaminergic adaptations and signaling to targets of the mesocorticolimbic system mediates drug reward. This review hopes to describe the projection-specific intrinsic characteristics of these subpopulations, the differential afferent inputs onto these VTA DA neuron subpopulations, and consolidate findings of drug-induced plasticity of VTA DA neurons and highlight the importance of future projection-based studies of this system.
Persistent adaptations in afferents to ventral tegmental dopamine neurons after opiate withdrawal.
DOI:10.1523/JNEUROSCI.0715-15.2015
URL
PMID:26180204
[本文引用: 4]

Protracted opiate withdrawal is accompanied by altered responsiveness of midbrain dopaminergic (DA) neurons, including a loss of DA cell response to morphine, and by behavioral alterations, including affective disorders. GABAergic neurons in the tail of the ventral tegmental area (tVTA), also called the rostromedial tegmental nucleus, are important for behavioral responses to opiates. We investigated the tVTA-VTA circuit in rats after chronic morphine exposure to determine whether tVTA neurons participate in the loss of opiate-induced disinhibition of VTA DA neurons observed during protracted withdrawal. In vivo recording revealed that VTA DA neurons, but not tVTA GABAergic neurons, are tolerant to morphine after 2 weeks of withdrawal. Optogenetic stimulation of tVTA neurons inhibited VTA DA neurons similarly in opiate-naive and long-term withdrawn rats. However, tVTA inactivation increased VTA DA activity in opiate-naive rats, but not in withdrawn rats, resembling the opiate tolerance effect in DA cells. Thus, although inhibitory control of DA neurons by tVTA is maintained during protracted withdrawal, the capacity for disinhibitory control is impaired. In addition, morphine withdrawal reduced both tVTA neural activity and tonic glutamatergic input to VTA DA neurons. We propose that these changes in glutamate and GABA inputs underlie the apparent tolerance of VTA DA neurons to opiates after chronic exposure. These alterations in the tVTA-VTA DA circuit could be an important factor in opiate tolerance and addiction. Moreover, the capacity of the tVTA to inhibit, but not disinhibit, DA cells after chronic opiate exposure may contribute to long-term negative affective states during withdrawal.Dopaminergic (DA) cells of the ventral tegmental area (VTA) are the origin of a brain reward system and are critically involved in drug abuse. Morphine has long been known to affect VTA DA cells via GABAergic interneurons. Recently, GABAergic neurons caudal to the VTA were discovered and named the tail of VTA (tVTA). Here, we show that tVTA GABA neurons lose their capacity to disinhibit, but not to inhibit, VTA DA cells after chronic opiate exposure. The failure of disinhibition was associated with a loss of glutamatergic input to DA neurons after chronic morphine. These findings reveal mechanisms by which the tVTA may play a key role in long-term negative affective states during opiate withdrawal.
Afferents to the GABAergic tail of the ventral tegmental area in the rat.
DOI:10.1002/cne.21983
URL
PMID:19235223
[本文引用: 2]

We previously showed that chronic psychostimulant exposure induces the transcription factor DeltaFosB in -aminobutyric acid (GABA)ergic neurons of the caudal tier of the ventral tegmental area (VTA). This subregion was defined as the tail of the VTA (tVTA). In the present study, we showed that tVTA can also be visualized by analyzing FosB/DeltaFosB response following acute cocaine injection. This induction occurs in GABAergic neurons, as identified by glutamic acid decarboxylase (GAD) expression. To characterize tVTA further, we mapped its inputs by using the retrograde tracers Fluoro-Gold or cholera toxin B subunit. Retrogradely labeled neurons were observed in the medial prefrontal cortex, the lateral septum, the ventral pallidum, the bed nucleus of the stria terminalis, the substantia innominata, the medial and lateral preoptic areas, the lateral and dorsal hypothalamic areas, the lateral habenula, the intermediate layers of the superior colliculus, the dorsal raphe, the periaqueductal gray, and the mesencephalic and pontine reticular formation. Projections from the prefrontal cortex, the hypothalamus, and the lateral habenula to the tVTA were also shown by using the anterograde tracer biotinylated dextran amine (BDA). We showed that the central nucleus of the amygdala innervates the anterior extent of the VTA but not the tVTA. Moreover, the tVTA mainly receives non-aminergic inputs from the dorsal raphe and the locus coeruleus. Although the tVTA has a low density of dopaminergic neurons, its afferents are mostly similar to those targeting the rest of the VTA. This suggests that the tVTA can be considered as a VTA subregion despite its caudal location. J. Comp. Neurol. 513:597-621, 2009. 2009 Wiley-Liss, Inc.
GIRK channels modulate opioid-induced motor activity in a cell type- and subunit-dependent manner.
DOI:10.1523/JNEUROSCI.5051-14.2015
URL
PMID:4420781
[本文引用: 1]

G-protein-gated inwardly rectifying K(+) (GIRK/Kir3) channel activation underlies key physiological effects of opioids, including analgesia and dependence. GIRK channel activation has also been implicated in the opioid-induced inhibition of midbrain GABA neurons and consequent disinhibition of dopamine (DA) neurons in the ventral tegmental area (VTA). Drug-induced disinhibition of VTA DA neurons has been linked to reward-related behaviors and underlies opioid-induced motor activation. Here, we demonstrate that mouse VTA GABA neurons express a GIRK channel formed by GIRK1 and GIRK2 subunits. Nevertheless, neither constitutive genetic ablation of Girk1 or Girk2, nor the selective ablation of GIRK channels in GABA neurons, diminished morphine-induced motor activity in mice. Moreover, direct activation of GIRK channels in midbrain GABA neurons did not enhance motor activity. In contrast, genetic manipulations that selectively enhanced or suppressed GIRK channel function in midbrain DA neurons correlated with decreased and increased sensitivity, respectively, to the motor-stimulatory effect of systemic morphine. Collectively, these data support the contention that the unique GIRK channel subtype in VTA DA neurons, the GIRK2/GIRK3 heteromer, regulates the sensitivity of the mouse mesolimbic DA system to drugs with addictive potential.
Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system.
DOI:10.1016/j.neuron.2008.01.022
URL
PMID:18341995
Magsci
[本文引用: 1]

The mesocorticolimbic dopamine system is essential for cognitive and emotive brain functions and is thus an important target in major brain diseases like schizophrenia, drug addiction, and attention deficit hyperactivity disorder. However, the cellular basis for the diversity in behavioral functions and associated dopamine-release pattern within the mesocorticolimbic system has remained unclear. Here, we report the identification of a type of dopaminergic neuron within the mesocorticolimbic dopamine system with unconventional fast-firing properties and small DAT/TH mRNA expression ratios that selectively projects to prefrontal cortex and nucleus accumbens core and medial shell as well as to basolateral amygdala. In contrast, well-described conventional slow-firing dopamine midbrain neurons only project to the lateral shell of the nucleus accumbens and the dorsolateral striatum. Among this dual dopamine midbrain system defined in this study by converging anatomical, electrophysiological, and molecular properties, mesoprefrontal dopaminergic neurons are unique, as only they do not possess functional somatodendritic Girk2-coupled dopamine D2 autoreceptors.
Projection-Specific modulation of dopamine neuron synapses by aversive and rewarding stimuli.
DOI:10.1016/j.neuron.2011.03.025
URL
PMID:21658580
Magsci
[本文引用: 1]

Midbrain dopamine (DA) neurons are not homogeneous but differ in their molecular properties and responses to external stimuli. We examined whether the modulation of excitatory synapses on DA neurons by rewarding or aversive stimuli depends on the brain area to which these DA neurons project. We identified DA neuron subpopulations in slices after injection of etrobeads into single target areas of adult mice and found differences in basal synaptic properties. Administration of cocaine selectively modified excitatory synapses on DA cells projecting to nucleus accumbens (NAc) medial shell while an aversive stimulus selectively modified synapses on DA cells projecting to medial prefrontal cortex. In contrast, synapses on DA neurons projecting to NAc lateral shell were modified by both rewarding and aversive stimuli, which presumably reflects saliency. These results suggest that the mesocorticolimbic DA system may be comprised of three anatomically distinct circuits, each modified by distinct aspects of motivationally relevant stimuli.Highlights? Dopamine neurons exhibit distinct basic synaptic properties ? In vivo cocaine modifies synapses on dopamine cells projecting to nucleus accumbens ? An aversive stimulus modifies synapses on mesocortical dopamine neurons ? Experience-dependent synaptic modifications in dopamine cells depend on their targets
Input-specific control of reward and aversion in the ventral tegmental area.
DOI:10.1038/nature11527
URL
PMID:23064228
Magsci
[本文引用: 3]

Abstract Ventral tegmental area (VTA) dopamine neurons have important roles in adaptive and pathological brain functions related to reward and motivation. However, it is unknown whether subpopulations of VTA dopamine neurons participate in distinct circuits that encode different motivational signatures, and whether inputs to the VTA differentially modulate such circuits. Here we show that, because of differences in synaptic connectivity, activation of inputs to the VTA from the laterodorsal tegmentum and the lateral habenula elicit reward and aversion in mice, respectively. Laterodorsal tegmentum neurons preferentially synapse on dopamine neurons projecting to the nucleus accumbens lateral shell, whereas lateral habenula neurons synapse primarily on dopamine neurons projecting to the medial prefrontal cortex as well as on GABAergic (纬-aminobutyric-acid-containing) neurons in the rostromedial tegmental nucleus. These results establish that distinct VTA circuits generate reward and aversion, and thereby provide a new framework for understanding the circuit basis of adaptive and pathological motivated behaviours.
The mesopontine rostromedial tegmental nucleus: An integrative modulator of the reward system.
DOI:10.1016/j.baga.2011.08.003
URL
PMID:3233474
Magsci
[本文引用: 2]

The mesopontine rostromedial tegmental nucleus (RMTg) is a newly discovered brain structure thought to profoundly influence reward-related pathways. The RMTg is prominently GABAergic, receives dense projections from the lateral habenula and projects strongly to the midbrain ventral tegmental area and substantia nigra compacta. It receives additional afferent connections from widespread brain structures and sends additional strong efferent connections to a number of non-dopaminergic brainstem structures and, to a lesser extent, the forebrain. Projection neurons of the RMTg have been shown to express Fos in response to aversive stimuli and/or reward omission and psychostimulant drug administration. This review will first recount how the RMTg was discovered and then describe in greater detail what is known about its neuroanatomical relationships, including afferent and efferent connections, neurotransmitters, and receptors. Finally, we will focus on what has been reported about its function.
Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells.
DOI:10.1038/npp.2010.190
URL
PMID:3055682
[本文引用: 2]

Abstract Recent findings have underlined the rostromedial tegmental nucleus (RMTg), a structure located caudally to the ventral tegmental area, as an important site involved in the mechanisms of aversion. RMTg contains aminobutyric acid neurons responding to noxious stimuli, densely innervated by the lateral habenula and providing a major inhibitory projection to reward-encoding midbrain dopamine (DA) neurons. One of the key features of drug addiction is the perseverance of drug seeking in spite of negative and unpleasant consequences, likely mediated by response suppression within neural pathways mediating aversion. To investigate whether the RMTg has a function in the mechanisms of addicting drugs, we studied acute effects of morphine, cocaine, the cannabinoid agonist WIN55212-2 (WIN), and nicotine on putative RMTg neurons. We utilized single unit extracellular recordings in anesthetized rats and whole-cell patch-clamp recordings in brain slices to identify and characterize putative RMTg neurons and their responses to drugs of abuse. Morphine and WIN inhibited both firing rate in vivo and excitatory postsynaptic currents (EPSCs) evoked by stimulation of rostral afferents in vitro, whereas cocaine inhibited discharge activity without affecting EPSC amplitude. Conversely, nicotine robustly excited putative RMTg neurons and enhanced EPSCs, an effect mediated by 7-containing nicotinic acetylcholine receptors. Our results suggest that activity of RMTg neurons is profoundly influenced by drugs of abuse and, as important inhibitory afferents to midbrain DA neurons, they might take place in the complex interplay between the neural circuits mediating aversion and reward.
Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse.
DOI:10.1038/npp.2011.302
URL
PMID:3306878
Magsci
[本文引用: 2]

Neuropsychopharmacology, the official publication of the American College of Neuropsychopharmacology, publishing the highest quality original research and advancing our understanding of the brain and behavior.
A dynamic role for GABA receptors on the firing pattern of midbrain dopaminergic neurons.
DOI:10.1152/jn.00204.2010
URL
PMID:20445035
[本文引用: 1]

Abstract Dopaminergic neurons are subject to a significant background GABAergic input in vivo. The presence of this GABAergic background might be expected to inhibit dopaminergic neuron firing. However, dopaminergic neurons are not all silent but instead fire in single-spiking and burst firing modes. Here we present evidence that phasic changes in the tonic activity of GABAergic afferents are a potential extrinsic mechanism that triggers bursts and pauses in dopaminergic neurons. We find that spontaneous single-spiking is more sensitive to activation of GABA receptors than phasic N-methyl-D-aspartate (NMDA)-mediated burst firing in rat slices (P15-P31). Because tonic activation of GABA(A) receptors has previously been shown to suppress burst firing in vivo, our results suggest that the activity patterns seen in vivo are the result of a balance between excitatory and inhibitory conductances that interact with the intrinsic pacemaking currents observed in slices. Using the dynamic clamp technique, we applied balanced, constant NMDA and GABA(A) receptor conductances into dopaminergic neurons in slices. Bursts could be produced by disinhibition (phasic removal of the GABA(A) receptor conductance), and these bursts had a higher frequency than bursts produced by the same NMDA receptor conductance alone. Phasic increases in the GABA(A) receptor conductance evoked pauses in firing. In contrast to NMDA receptor, application of constant AMPA and GABA(A) receptor conductances caused the cell to go into depolarization block. These results support a bidirectional mechanism by which GABAergic inputs, in balance with NMDA receptor-mediated excitatory inputs, control the firing pattern of dopaminergic neurons.
Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal.
DOI:10.1016/j.neuron.2014.04.030
URL
PMID:24857021
Magsci
[本文引用: 5]

GABA release from interneurons in VTA, projections from the nucleus accumbens (NAc), and rostromedial tegmental nucleus (RMTg) was selectively activated in rat brain slices. The inhibition induced by m-opioid agonists was pathway dependent. Morphine induced a 46% inhibition of IPSCs evoked from the RMTg, 18% from NAc, and IPSCs evoked from VTA interneurons were almost insensitive (11% inhibition). In vivo morphine treatment resulted in tolerance to the inhibition of RMTg, but not local interneurons or NAc, inputs. One common sign of opioid withdrawal is an increase in adenosine-dependent inhibition. IPSCs evoked from the NAc were potently inhibited by activation of presynaptic adenosine receptors, whereas IPSCs evoked from RMTg were not changed. Blockade of adenosine receptors selectively increased IPSCs evoked from the NAc during morphine withdrawal. Thus, the acute action of opioids, the development of tolerance, and the expression of withdrawal are mediated by separate GABA afferents to dopamine neurons.
Opioid-Sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons.
DOI:10.1523/JNEUROSCI.4570-11.2011
URL
PMID:22131433
[本文引用: 3]

Opioids increase dopamine release in the brain through inhibition of GABA-A IPSCs onto dopamine cells. Immunolabeling indicates that GABA neurons in the rostromedial tegmental nucleus (RMTg), also known as the tail of the ventral tegmental area, send a dense projection to midbrain dopamine neurons stain for μ-opioid receptors. There is however, little functional evidence that these neurons play a role in the opioid-dependent increase in dopamine neuron activity. The present study used retrograde tracers injected into the ventral tegmental area and substantia nigra (VTA/SN) to identify RMTg neurons that project to the VTA/SN. Whole-cell current-clamp and cell-attached recordings from labeled RMTg neurons were performed in sagittal slices from rat. The rhythmic spontaneous firing rate of RMTg neurons was decreased and the membrane potential was hyperpolarized in response to application of μ-opioid agonist DAMGO. Agonists that act at κ- and δ-opioid receptors (U69593 and DPDPE) failed to hyperpolarize RMTg neurons. Whole-cell recordings made in dopamine neurons revealed rhythmic, large amplitude spontaneous IPSCs that had a similar frequency, pattern and opioid sensitivity to the firing of RMTg neurons. In addition, electrical and channelrhodopsin-2 stimulation within the RMTg evoked GABA-A IPSCs in dopamine neurons that were inhibited by μ-opioid agonists DAMGO, but not κ- and δ-opioid agonists. Thus, this study demonstrates functional connection from the RMTg to the VTA/SN mediated by a dense, opioid-sensitive GABA innervation, and that the RMTg is a key structure in the μ-opioid receptor-dependent regulation of dopamine neurons.
Lateral habenula as a source of negative reward signals in dopamine neurons.
DOI:10.1038/nature05860
URL
PMID:17522629
[本文引用: 2]

Abstract Midbrain dopamine neurons are key components of the brain's reward system, which is thought to guide reward-seeking behaviours. Although recent studies have shown how dopamine neurons respond to rewards and sensory stimuli predicting reward, it is unclear which parts of the brain provide dopamine neurons with signals necessary for these actions. Here we show that the primate lateral habenula, part of the structure called the epithalamus, is a major candidate for a source of negative reward-related signals in dopamine neurons. We recorded the activity of habenula neurons and dopamine neurons while rhesus monkeys were performing a visually guided saccade task with positionally biased reward outcomes. Many habenula neurons were excited by a no-reward-predicting target and inhibited by a reward-predicting target. In contrast, dopamine neurons were excited and inhibited by reward-predicting and no-reward-predicting targets, respectively. Each time the rewarded and unrewarded positions were reversed, both habenula and dopamine neurons reversed their responses as the bias in saccade latency reversed. In unrewarded trials, the excitation of habenula neurons started earlier than the inhibition of dopamine neurons. Furthermore, weak electrical stimulation of the lateral habenula elicited strong inhibitions in dopamine neurons. These results suggest that the inhibitory input from the lateral habenula plays an important role in determining the reward-related activity of dopamine neurons.
Two types of dopamine neuron distinctly convey positive and negative motivational signals.
Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABAA receptors in vivo.
DOI:10.1016/S0306-4522(98)00355-8
URL
PMID:10199614
[本文引用: 1]

These data demonstrate that dopaminergic neurons of the substantia nigra pars compacta are inhibited by electrical stimulation of striatum, globus pallidus, and the projection neurons of substantia nigra pars reticulata in vivo . This inhibition appears to be mediated via the GABA A receptor subtype, and all three GABAergic afferents studied appear to possess inhibitory presynaptic GABA B autoreceptors that are active under physiological conditions in vivo .
Anterior and posterior parts of the rat ventral tegmental area and the rostromedial tegmental nucleus receive topographically distinct afferents from the lateral habenular complex.
DOI:10.1002/cne.24200
URL
PMID:28295296
[本文引用: 1]

Abstract That activation of the reward system involves increased activity of dopaminergic (DA) neurons in the ventral tegmental area (VTA) is widely accepted. In contrast, the lateral habenular complex (LHb), which is known as the center of the anti-reward system, directly and indirectly inhibits DA neurons in the VTA. The VTA, however, is not a homogenous entity with major functional differences between its anterior (aVTA) and posterior (pVTA) regions. It is not precisely known, whether habenular input to the aVTA, pVTA, and the newly recognized rostromedial tegmental nucleus (RMTg) are similarily or differently organized. Consequently, the present investigation addressed the connections between LHb and aVTA, pVTA, and RMTg using retrograde and anterograde tracing techniques in the rat. Our experiments disclosed strictly reciprocal and conspicuously focal interconnections between LHbM (LHbMPc/LHbMC) and PN, as well as between RLi and LHbLO. In addition we found that LHb inputs to the aVTA are dorsoventrally ordered. Dorsal parts of the aVTA receive afferents from LHbL and LHbM, whereas ventral parts of the aVTA are preferentially targeted by the LHbM. LHb afferents to the pVTA are distinct from those to the RMTg, given that the RMTg is primarily innervated from the LHbL, whereas pVTA receives afferents from LHbM and LHbL. These data indicate the existence of two separate pathways from the LHb to the VTA, a direct and an indirect one, which may subserve distinct biological functions This article is protected by copyright. All rights reserved.
Role of dopamine neurons in reward and aversion: A synaptic plasticity perspective.
DOI:10.1016/j.neuron.2015.04.015
URL
PMID:26050034
[本文引用: 3]

Pignatelli and Bonci review emerging concepts on synaptic plasticity of dopaminergic neurons, with respect to neural processing and coding of both rewarding and aversive stimuli.
Morphine-induced place preference: Involvement of cholinergic receptors of the ventral tegmental area.
DOI:10.1016/j.ejphar.2007.01.081
URL
PMID:17336285
[本文引用: 1]

In the present study, the effects of intra-ventral tegmental area injections of cholinergic agents on morphine-induced conditioned place preference were investigated by using an unbiased 3-day schedule of place conditioning design in rats. The conditioning treatments with subcutaneous injections of morphine (0.5–7.502mg/kg) induced a significant dose-dependent conditioned place preference for the drug-associated place. Intra-ventral tegmental area injection of an anticholinesterase, physostigmine (2.502and 502μg/rat) or nicotinic acetylcholine receptor agonist, nicotine (0.5 and 102μg/rat) with an ineffective dose of morphine (0.502mg/kg) elicited a significant conditioned place preference. Furthermore, intra-ventral tegmental area administration of muscarinic acetylcholine receptor antagonist, atropine (1–402μg/rat) or nicotinic acetylcholine receptor antagonist, mecamylamine (5 and 7.502μg/rat) dose-dependently inhibited the morphine (502mg/kg)-induced place preference. Atropine or mecamylamine reversed the effect of physostigmine or nicotine on morphine response respectively. The injection of physostigmine, but not atropine, nicotine or mecamylamine, into the ventral tegmental area alone produced a significant place aversion. Moreover, intra-ventral tegmental area administration of the higher doses of physostigmine or atropine, but not nicotine or mecamylamine decreased the locomotor activity. We conclude that muscarinic and nicotinic acetylcholine receptors in the ventral tegmental area may critically mediate the rewarding effects of morphine.
The brain reward circuitry in mood disorders.
DOI:10.1038/nrn3381
Magsci
[本文引用: 1]

Mood disorders are common and debilitating conditions characterized in part by profound deficits in reward-related behavioural domains. A recent literature has identified important structural and functional alterations within the brain's reward circuitry-particularly in the ventral tegmental area-nucleus accumbens pathway-that are associated with symptoms such as anhedonia and aberrant reward-associated perception and memory. This Review synthesizes recent data from human and rodent studies from which emerges a circuit-level framework for understanding reward deficits in depression. We also discuss some of the molecular and cellular underpinnings of this framework, ranging from adaptations in glutamatergic synapses and neurotrophic factors to transcriptional and epigenetic mechanisms.
Response of the tail of the ventral tegmental area to aversive stimuli.
DOI:10.1038/npp.2016.139
URL
PMID:27468916
[本文引用: 1]

Abstract The GABAergic tail of the ventral tegmental area (tVTA), also named rostromedial tegmental nucleus (RMTg), exerts an inhibitory control on dopamine neurons of the VTA and substantia nigra. The tVTA has been implicated in avoidance behaviors, response to drugs of abuse, reward prediction error and motor functions. Stimulation of the lateral habenula inputs to the tVTA, or of the tVTA itself, induces avoidance behaviors, which suggests a role of the tVTA in processing aversive information. Our aim was to test the impact of aversive stimuli on the molecular recruitment of the tVTA, and the behavioral consequences of tVTA lesions. In rats, we assessed Fos response to lithium chloride (LiCl), carboline, naloxone, lipopolysaccharide (LPS), inflammatory pain, neuropathic pain, foot-shock, restraint stress, forced swimming, predator odor, and opiate withdrawal. We also determined the effect of tVTA bilateral ablation on physical signs of opiate withdrawal, and on LPS- and LiCl-induced conditioned taste aversion (CTA). Naloxone-precipitated opiate withdrawal induced Fos in mu-opioid receptor positive (15%) and negative (85%) tVTA cells, suggesting the presence of both direct and indirect mechanisms in tVTA recruitment during withdrawal. However, tVTA lesion did not impact physical signs of opiate withdrawal. Fos induction was also present with repeated, but not single, foot-shock delivery. However, such induction was mostly absent with other aversive stimuli. Moreover, tVTA ablation had no impact on CTA. While stimulation of the tVTA favors avoidance behaviors, present findings suggest that this structure may be important to the response to some, but not all, aversive stimuli.Neuropsychopharmacology accepted article preview online, 29 July 2016. doi:10.1038/npp.2016.139.
BOLD responses to negative reward prediction errors in human habenula.
Although positive reward prediction error, a key element in learning that is signaled by dopamine cells, has been extensively studied, little is known about negative reward prediction errors in humans. Detailed animal electrophysiology shows that the habenula, an integrative region involved in many processes including learning, reproduction, and stress responses, also encodes negative reward-related events such as negative reward prediction error signals. In humans, however, the habenula's extremely small size has prevented direct assessments of its function. We developed a method to functionally locate and study the habenula in humans using fMRI, based on the expected reward-dependent response phenomenology of habenula and striatum and, we provide conclusive evidence for activation in human habenula to negative reward prediction errors.
The antero-posterior heterogeneity of the ventral tegmental area.
DOI:10.1016/j.neuroscience.2014.09.025
URL
PMID:25241061
[本文引用: 2]

The ventral tegmental area (VTA) is a brain region processing salient sensory and emotional information, controlling motivated behaviors, natural or drug-related reward, reward-related learning, mood, and participating in their associated psychopathologies. Mostly studied for its dopamine neurons, the VTA also includes functionally important GABA and glutamate cell populations. Behavioral evidence supports the presence of functional differences between the anterior VTA (aVTA) and the posterior VTA (pVTA), which is the topic of this review. This antero-posterior heterogeneity concerns locomotor activity, conditioned place preference and intracranial self-administration, and can be seen in response to ethanol, acetaldehyde, salsolinol, opioids including morphine, cholinergic agonists including nicotine, cocaine, cannabinoids and after local manipulation of GABA and serotonin receptors. It has also been observed after viral-mediated manipulation of GluR1, phospholipase C (PLC) and cAMP response element binding protein (CREB) expression, with impact on reward and aversion-related responses, on anxiety and depression-related behaviors and on pain sensitivity. In this review, the substrates potentially underlying these aVTA/pVTA differences are discussed, including the VTA sub-nuclei and the heterogeneity in connectivity, cell types and molecular characteristics. We also review the role of the tail of the VTA (tVTA), or rostromedial tegmental nucleus (RMTg), which may also participate to the observed antero-posterior heterogeneity of the VTA. This region, partly located within the pVTA, is an inhibitory control center for dopamine activity. It controls VTA and substantia nigra dopamine cells, thus exerting a major influence on basal ganglia functions. This review highlights the need for a more comprehensive analysis of VTA heterogeneity.
A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward.
DOI:10.1016/j.neuron.2013.08.023
URL
PMID:3873746
Magsci
[本文引用: 1]

Stamatakis et02al. demonstrate that a unique population of ventral tegmental area neurons expresses GABAergic and dopaminergic markers and project to the lateral habenula. Functionally, this projection inhibits postsynaptic lateral habenula neurons, via released GABA, to promote reward.
Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance.
DOI:10.1038/nn.3145
URL
PMID:3411914
Magsci
[本文引用: 1]

Lateral habenula (LHb) projections to the ventral midbrain, including the rostromedial tegmental nucleus (RMTg), convey negative reward-related information, but the behavioral ramifications of selective activation of this pathway remain unexplored. We found that exposure to aversive stimuli in mice increased LHb excitatory drive onto RMTg neurons. Furthermore, optogenetic activation of this pathway promoted active, passive and conditioned behavioral avoidance. Thus, activity of LHb efferents to the midbrain is aversive but can also serve to negatively reinforce behavioral responding.
Electrophysiological characterization of GABAergic neurons in the ventral tegmental area.
Muscarinic cholinergic receptor antagonists in the VTA and RMTg have opposite effects on morphine-induced locomotion in mice.
DOI:10.1016/j.bbr.2017.01.039
URL
PMID:28143769
[本文引用: 4]

The ventral tegmental area (VTA) and the rostromedial tegmental nucleus (RMTg) each contribute to opiate reward and each receive inputs from the laterodorsal tegmental and pedunculopontine tegmental nuclei, the two principle brainstem cholinergic cell groups. We compared the contributions of VTA or RMTg muscarinic cholinergic receptors to locomotion induced by morphine infusions into the same sites. VTA co-infusion of atropine completely blocked VTA morphine-induced locomotion providing additional support for the important role of VTA muscarinic cholinergic receptors in the stimulant effects of opiates. By contrast, RMTg co-infusion of atropine increased RMTg morphine-induced locomotion. Furthermore, RMTg co-infusion of the M3-selective antagonist 4-DAMP, but not the M4-selective antagonist Tropicamide, strongly increased RMTg morphine-induced locomotion. RMTg infusions of 4-DAMP, but not of Tropicamide, by themselves strongly increased drug-free locomotion. Muscarinic cholinergic receptors in the RMTg thus also contribute to the stimulant effects of morphine, but in a way opposite to those in VTA. We suggest that the net effect of endogenous cholinergic input to the RMTg on drug-free and on RMTg morphine-induced locomotion is inhibitory.
M5 muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice.
DOI:10.1371/journal.pone.0027538
URL
PMID:22102904
[本文引用: 1]

Abstract Opiates, like other addictive drugs, elevate forebrain dopamine levels and are thought to do so mainly by inhibiting GABA neurons near the ventral tegmental area (VTA), in turn leading to a disinhibition of dopamine neurons. However, cholinergic inputs from the laterodorsal (LDT) and pedunculopontine (PPT) tegmental nucleus to the VTA and substantia nigra (SN) importantly contribute, as either LDT or PPT lesions strongly attenuate morphine-induced forebrain dopamine elevations. Pharmacological blockade of muscarinic acetylcholine receptors in the VTA or SN has similar effects. M63 muscarinic receptors are the only muscarinic receptor subtype associated with VTA and SN dopamine neurons. Here we tested the contribution of M63 muscarinic receptors to morphine-induced dopamine elevations by measuring nucleus accumbens dopamine efflux in response to intra-VTA morphine infusion using in vivo chronoamperometry. Intra-VTA morphine increased nucleus accumbens dopamine efflux in urethane-anesthetized wildtype mice starting at 10 min after infusion. These increases were absent in M63 knockout mice and were similarly blocked by pre-treatment with VTA scopolamine in wildtype mice. Furthermore, in wildtype mice electrical stimulation of the PPT evoked an initial, short-lasting increase in striatal dopamine efflux, followed 5 min later by a second prolonged increase in dopamine efflux. In M63 knockout mice, or following systemic pre-treatment with scopolamine in wildtype mice, the prolonged increase in striatal dopamine efflux was absent. The time course of increased accumbal dopamine efflux in wildtype mice following VTA morphine was consistent with both the prolonged M63-mediated excitation of striatal dopamine efflux following PPT electrical stimulation and accumbal dopamine efflux following LDT electrical stimulation. Therefore, M63 receptors appear critical for prolonged PPT excitation of dopamine efflux and for dopamine efflux induced by intra-VTA morphine.
Supplemental morphine infusion into the posterior ventral tegmentum extends the satiating effects of self-administered intravenous heroin.
DOI:10.1016/j.pbb.2015.04.006
URL
PMID:25913296
[本文引用: 1]

61Ventral tegmental morphine increases the pauses between responses for i.v. heroin.61Posterior ventral tegmental infusions are most effective.61Ventral tegmental morphine appears to prolong a post-injection heroin satiety state.
Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons.
DOI:10.1016/j.neubiorev.2017.09.022
URL
PMID:28951251

Berkeley Electronic Press Selected Works
A causal link between prediction errors, dopamine neurons and learning.
DOI:10.1038/nn.3413
URL
PMID:23708143

Situations where rewards are unexpectedly obtained or withheld represent opportunities for new learning. Often, this learning includes identifying cues that predict reward availability. Unexpected rewards strongly activate midbrain dopamine neurons. This phasic signal is proposed to support learning about antecedent cues by signaling discrepancies between actual and expected outcomes, termed a reward prediction error. However, it is unknown whether dopamine neuron prediction error signaling and cue-reward learning are causally linked. To test this hypothesis, we manipulated dopamine neuron activity in rats in two behavioral procedures, associative blocking and extinction, that illustrate the essential function of prediction errors in learning. We observed that optogenetic activation of dopamine neurons concurrent with reward delivery, mimicking a prediction error, was sufficient to cause long-lasting increases in cue-elicited reward-seeking behavior. Our findings establish a causal role for temporally-precise dopamine neuron signaling in cue-reward learning, bridging a critical gap between experimental evidence and influential theoretical frameworks.
Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine-induced locomotion.
DOI:10.1111/ejn.13237
URL
PMID:26990801
[本文引用: 3]

Opioids induce rewarding and locomotor effects by inhibiting rostromedial tegmental GABA neurons that express opioid and nociceptin receptors. These GABA neurons then strongly inhibit dopamine neurons. Opioid-induced reward, locomotion and dopamine release also depend on pedunculopontine and laterodorsal tegmental cholinergic and glutamate neurons, many of which project to and activate ventral tegmental area dopamine neurons. Here we show that laterodorsal tegmental and pedunculopontine cholinergic neurons project to both rostromedial tegmental nucleus and ventral tegmental area, and that M4 muscarinic receptors are co-localized with opioid receptors associated with rostromedial tegmental GABA neurons. To inhibit or excite rostromedial tegmental GABA neurons, we utilized adeno-associated viral vectors and DREADDs to express designed muscarinic receptors (M4D or M3D respectively) in GAD2::Cre mice. In M4D-expressing mice, clozapine-N-oxide increased morphine-induced, but not vehicle-induced, locomotion. In M3D-expressing mice, clozapine-N-oxide blocked morphine-induced, but not vehicle-induced, locomotion. We propose that cholinergic inhibition of rostromedial tegmental GABA neurons via M4 muscarinic receptors facilitates opioid inhibition of the same neurons. This model explains how mesopontine cholinergic systems and muscarinic receptors in the rostromedial tegmental nucleus and ventral tegmental area are important for dopamine-dependent and dopamine-independent opioid-induced rewards and locomotion.
Cholinergic control of morphine- induced locomotion in rostromedial tegmental nucleus versus ventral tegmental area sites.
DOI:10.1111/ejn.12279
URL
PMID:23773170
Magsci
[本文引用: 2]

M5 muscarinic acetylcholine receptors expressed on ventral tegmental dopamine (DA) neurons are needed for opioid activation of DA outputs. Here, the M5 receptor gene was bilaterally transfected into neurons in the ventral tegmental area (VTA) or the adjacent rostromedial tegmental nucleus (RMTg) in mice by means of a Herpes simplex viral vector (HSV) to increase the effect of endogenous acetylcholine. Three days after HSV-M5 gene infusion in VTA sites, morphine-induced locomotion more than doubled at two doses, while saline-induced locomotion was unaffected. When the HSV-M5 gene was infused into the adjacent RMTg, morphine-induced locomotion was strongly inhibited. The sharp boundary between these opposing effects was found where tyrosine hydroxylase (TH) and cholinesterase labelling decreases (4.00 mm posterior to bregma). The same HSV-M5 gene transfections in M5 knockout mice induced even stronger inhibitory behavioural effects in RMTg but more variability in VTA sites due to stereotypy. The VTA sites where HSV-M5 increased morphine-induced locomotion receive direct inputs from many RMTg GAD-positive neurons, and from pontine ChAT-positive neurons, as shown by cholera-toxin B retrograde tracing. Therefore, morphine-induced locomotion was decreased by M5 receptor gene expression in RMTg GABA neurons that directly inhibit VTA DA neurons. Conversely, enhancing M5 receptor gene expression on VTA DA neurons increased morphine-induced locomotion via cholinergic inputs.
ATLAS on substance use (2010): Resources for the prevention and treatment of substance use disorders. World Health Organ.